INTRODUCTION
Nature has developed a multitude of hierarchical biological components with fascinating and complex superstructures, such as protein, sugar and DNA, etc., to perform information storage and transport, pharmaceutical and toxicological activities, and localized chemical transformations.[1] Among these macromolecular components, chirality is an essential and basic characteristic. For example, D-sugars are the main components of DNA and the secondary R-helix structures are also prevalent in proteins.[2] Scientists have been inspired by nature and developed many pioneering concepts with respect to chirality, such as ‘optical activity’, ‘asymmetry’, ‘enantiomers’ and ‘chirality’ since Pasteur's resolution of racemic tartaric acid.[3] In later years, as chirality was found in various biomimetic and artificial systems, it became highly important not only for fundamental science but also for numerous practical applications, such as molecular and chiral recognition, chiral sensors, chiroptical switches and asymmetric catalysis.[4]
In recent years, tremendously increasing efforts have been invested to understand the internal mechanisms and external influence factors of chirality. Research on the mechanism of chirality induction, transfer and amplification plays an important role in asymmetric synthesis and further applications of chirality.[5] Due to the nature of structural diversity, chirality is hierarchical and can be observed at a broad range of length scales from subatomic and molecular to supramolecular, nanoscopic, macroscopic and galactic levels (Scheme 1).[6] For instance, only left-handed helical neutrinos are found in physics, which indicates that chirality is related to parity conservation in atom. At a molecular level, many chiral molecules exist in natural (amino acids) and synthetic compounds. These molecules contain the primary chiral structures generated by the asymmetric chemical connections of atoms. Further investigations of such molecular chirality can be very important as it can provide general guidance for researchers to design targeted drugs and functional materials.[7] Furthermore, chirality is also expressed at the macromolecular or supramolecular level, as seen in the helical structures of DNA, secondary structures of protein, and tobacco mosaic virus. Even macroscopic living systems, such as cucumber vines, exhibit chiral features.[8] On a light-year scale, our galaxy system is also chiral and the spiral movement of the cosmic nebula has persisted throughout the immense duration of galactic history.

However, these chiral features at different length scales are inseparable and interrelated, which constitute our whole biological systems and macroscopic world. For example, the primary molecular chirality originates from the asymmetric arrangement of atoms in space around a centre (point chirality), axis (axial chirality) or plane (planar chirality), while the higher scale of chirality may be ascribed to the physical or chemical combinations between various fundamental chiral components.[9] More importantly, further understanding of the chirality in nature and the development of asymmetric assembly allow us to design targeted chiral superstructures at different levels. These in turn could allow scientists to investigate the mechanisms of chirality induction, transfer, and amplification from low-level scale into higher hierarchies. As a result, these complementary and hierarchical processes may lead to deeper understanding of the chirality in biomacromolecules, and even in living biological systems.
Among various levels, chirality at supramolecular level is particularly important because it is prevalent in the complex components in living organisms.[10] In addition, with the rapid developments of supramolecular chemistry and self-assembly technology, supramolecular chirality has received significant attention and experienced significant advancements under the efforts of researchers.[11] In general, supramolecular chirality is constructed through the nonsymmetric arrangement of building blocks via weak noncovalent interactions, such as π-π stacking, hydrogen bonding, dipole-dipole interactions and host-guest interactions.[12−15] When the building blocks prefer an asymmetric packing mode during self-assembly, a chiral architecture will be formed at supramolecular scale. Compared with molecular chirality, supramolecular chirality is generally more dynamic and stimuli-responsive due to the presence of weak noncovalent interactions.[16] Furthermore, it is not required that all building blocks are chiral. In other words, these building blocks can be chiral, achiral or a combination of both chiral and achiral components. There are two main factors that determine whether a superstructure has supramolecular chirality, (1) one or more of the components are chiral and interact via non-covalent bonds such that the assemblies have determined supramolecular chirality; (2) the achiral components associate in noncovalent interactions but the assemblies adopt predominantly one-handed helicity. This means that the supramolecular chirality depends both on the weak noncovalent interactions and the assembly behavior of the building blocks.
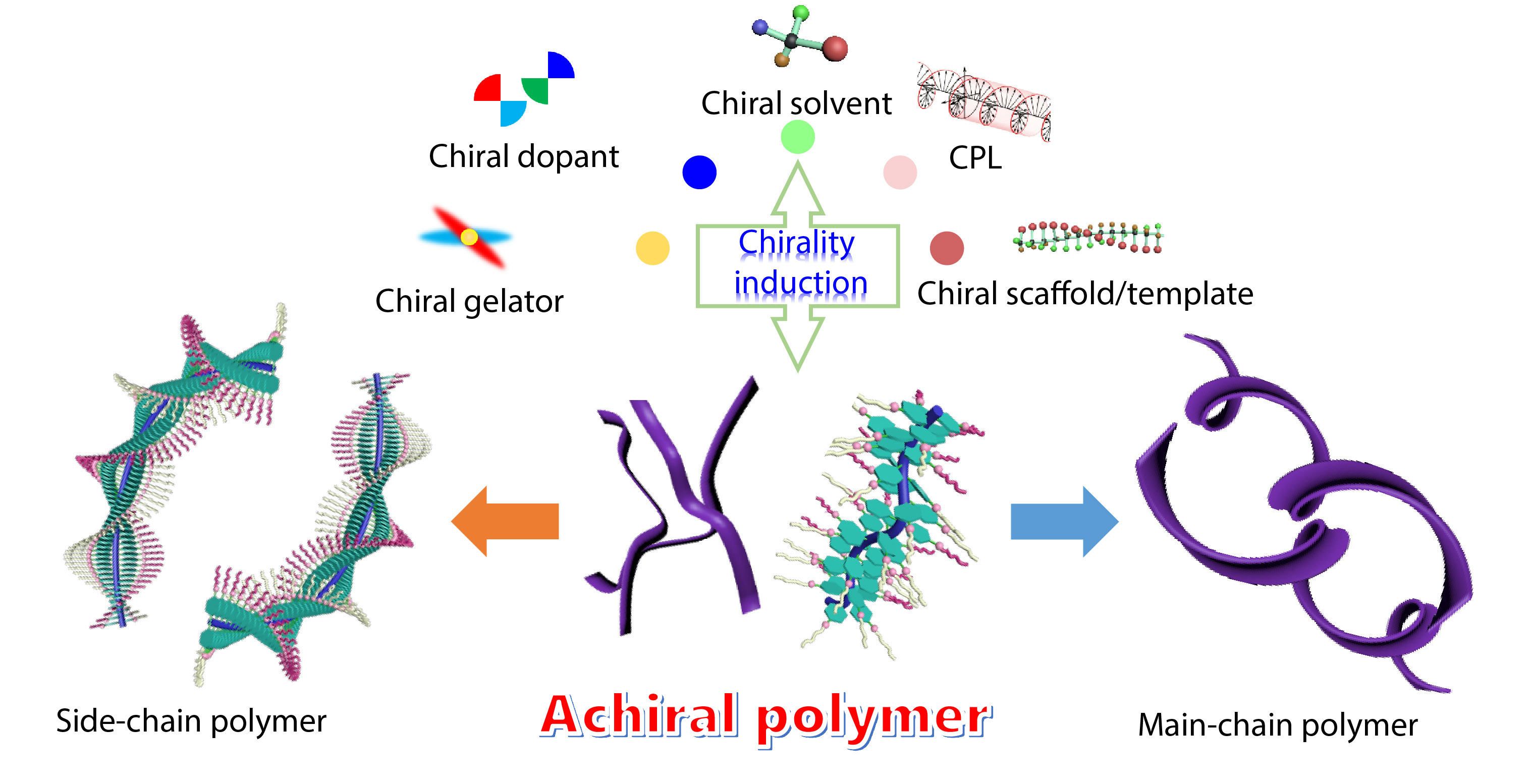
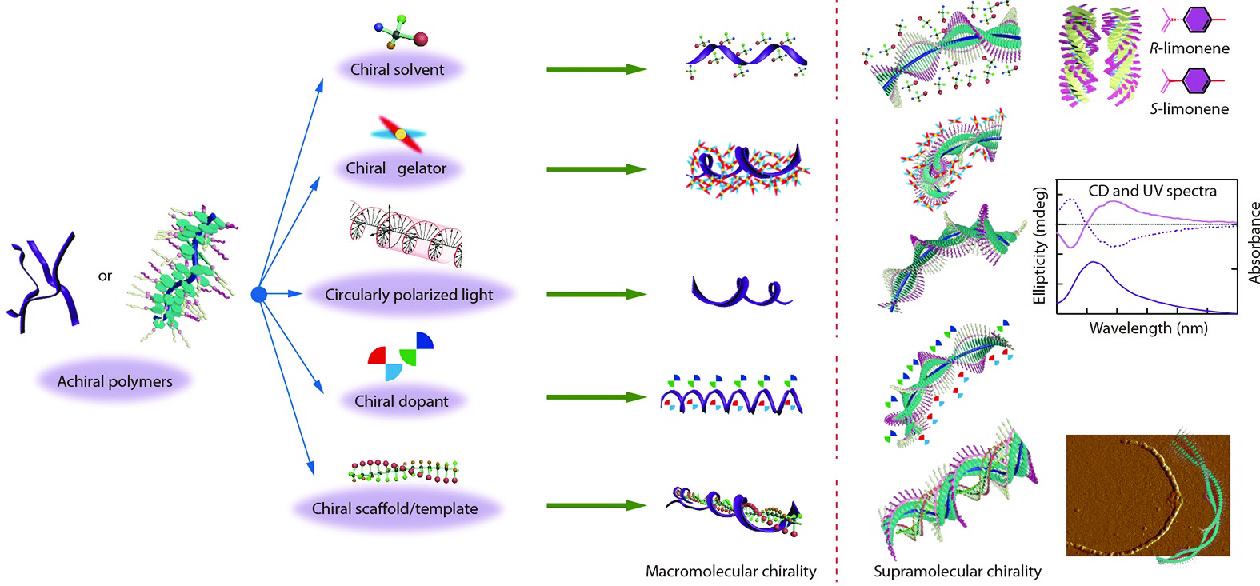
Generally, most macromolecules or polymers possess supramolecular chirality by directly using chiral building blocks in the process of self-assembly.[17−19] This kind of chirality is attributed to straightforward chirality induction biases. However, it requires tedious procedures for designing and synthesizing targeted chiral monomers/catalysts with high enantiopurity (ee), or complex asymmetric polymerization processes. Therefore, supramolecular chirality produced from achiral building blocks has received much attention in recent years. Nowadays, some advanced induction strategies, including but not limited to induction by chiral solvation, chiral dopant, chiral gelator, circularly polarized light (CPL) and chiral scaffold/template[20−23] have been developed and successfully implemented to construct supramolecular chirality from achiral building blocks, which have also been studied by our group as shown in Scheme 2. The process of induction strategy usually involves chirality transfer from a chiral component to an achiral one followed by the ordered asymmetric extension over the entire system. An important mechanism in chirality induction is the chirality transfer from a chiral source to achiral polymers, by which chiral ‘information’ can be amplified by achiral polymers to construct supramolecular chirality in an achiral polymer system.
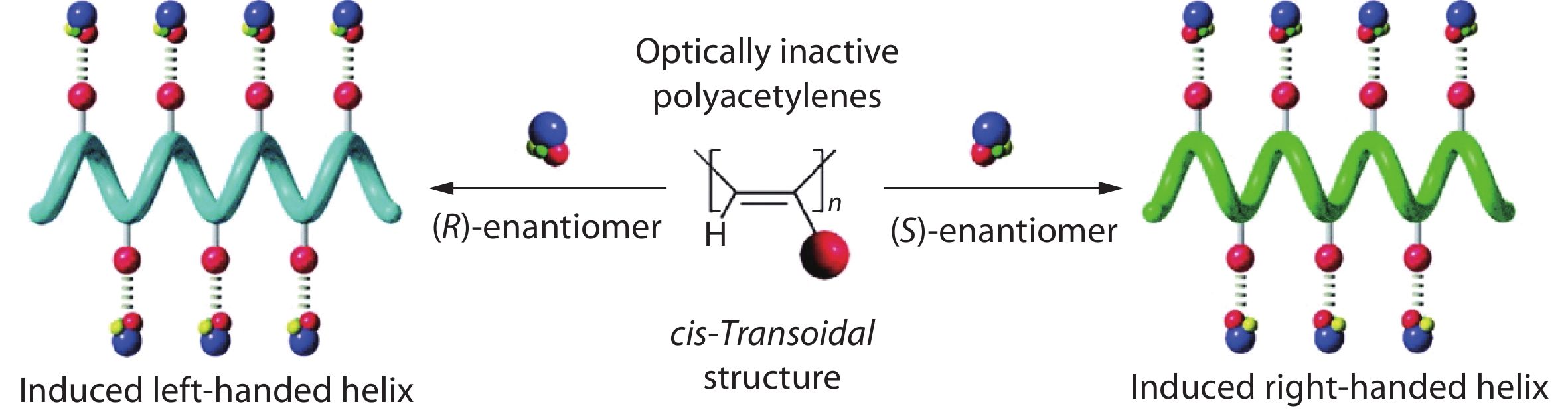
Induced supramolecular chirality is of particular interest and importance in polymer systems due to its broad range of applications. The induced chirality in polymer systems can be constructed by the supramolecular assembly of main-chain or side-chain chiral/achiral polymers. In 1995, Yashima et al. reported the first example of induced chirality in achiral poly((4-carboxyphenyl)acetylene) by chiral amines through acid-base interactions and systematically investigated the formation process of one-handed helices (Fig. 1).[5,24] Fujiki et al. also reported the chirality transfer and amplification from chiral molecules to achiral polysilylene (PSi) aggregates through weak hydrogen-bonding interaction.[25] To date, several methods, such as chiral solvation, chiral gelation, CPL and chiral doping, have been applied to induce chirality in polymer systems.[26−29] It should be noted that in side-chain polymers, the induced chirality is attributed to asymmetric packing of building blocks with right/left-handed direction, while for main-chain polymers, the induced chirality is usually attributed to the helix chirality of the main-chain backbone. The induction strategy showed convincing results in inducing chirality in many achiral polymers, including poly(n-hexyl isocyanate),[30] polysilanes,[31] polyfluorene analogs,[32] polydiacetylene and polyacetylenes.[33]
In the past several years, our group has devoted great efforts to developing induction strategies for constructing supramolecular chirality in polymers, aiming to expand the scope of such strategies. Significant efforts have also been devoted in studying the inner mechanism and self-assembly manner. In this feature article, we summarize our recent work on the construction of supramolecular chirality in polymer systems, mainly discussing the chirality induction, transfer, and application in achiral systems. To induce supramolecular chirality in polymer systems, different methods of chirality induction have been used, including chiral solvation, chiral gelation, CPL, chiral doping and chiral scaffold. Several examples of achiral main-chain and side-chain polymers with supramolecular chirality constructed by the induction strategy will be specifically highlighted. This feature article aims to stimulate interests from researchers in supramolecular chirality and make contributions to extending the research scope of this strategy to further advance the development of chiral chemistry.
CHIRALITY INDUCTION AND TRANSFER
Chiral Solvent
Chiral solvation is a simple and effective method for inducing chirality in achiral substances, which is commonly used in small molecules, oligomers and polymer systems. As early as 1993, Green et al. first demonstrated that a change in macromolecular conformation can be observed in an achiral poly(n-hexyl isocyanate) (PHIC) driven by a minute chiral solvation energy.[34] The obvious optical activity can be confirmed in the circular dichroism (CD) spectrum when the achiral PHIC was dissolved in several chiral solvents. This novel work shows that chirality transfer can be achieved by simply adding the achiral PHICs into non-racemic solvents. Motivated by this pioneering study, chiral solvation has been successfully used to induce chirality in many achiral polymers in aggregation state, such as polysilanes,[25] polyfluorenes[32] and polyacetylenes.[35]
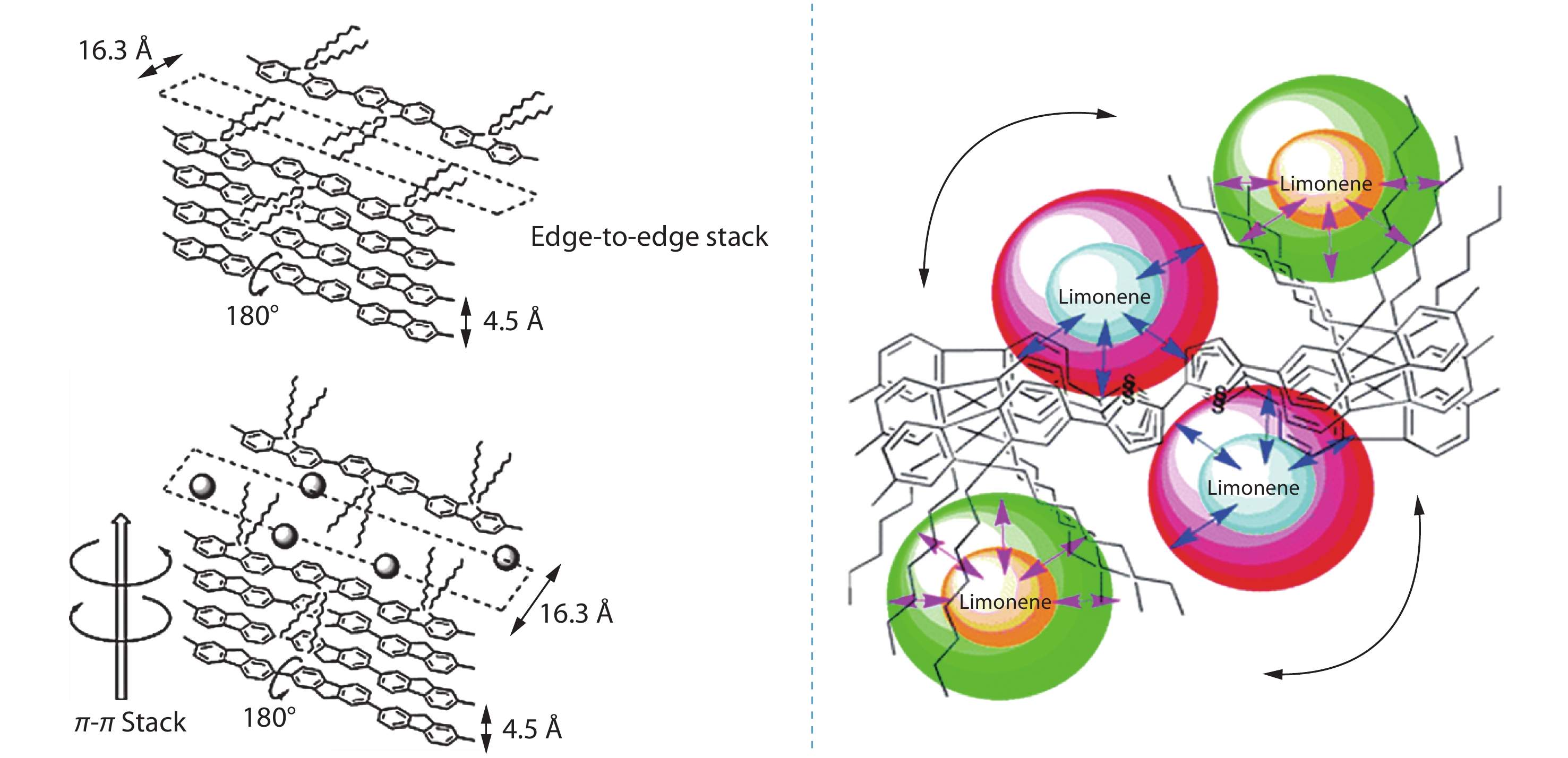
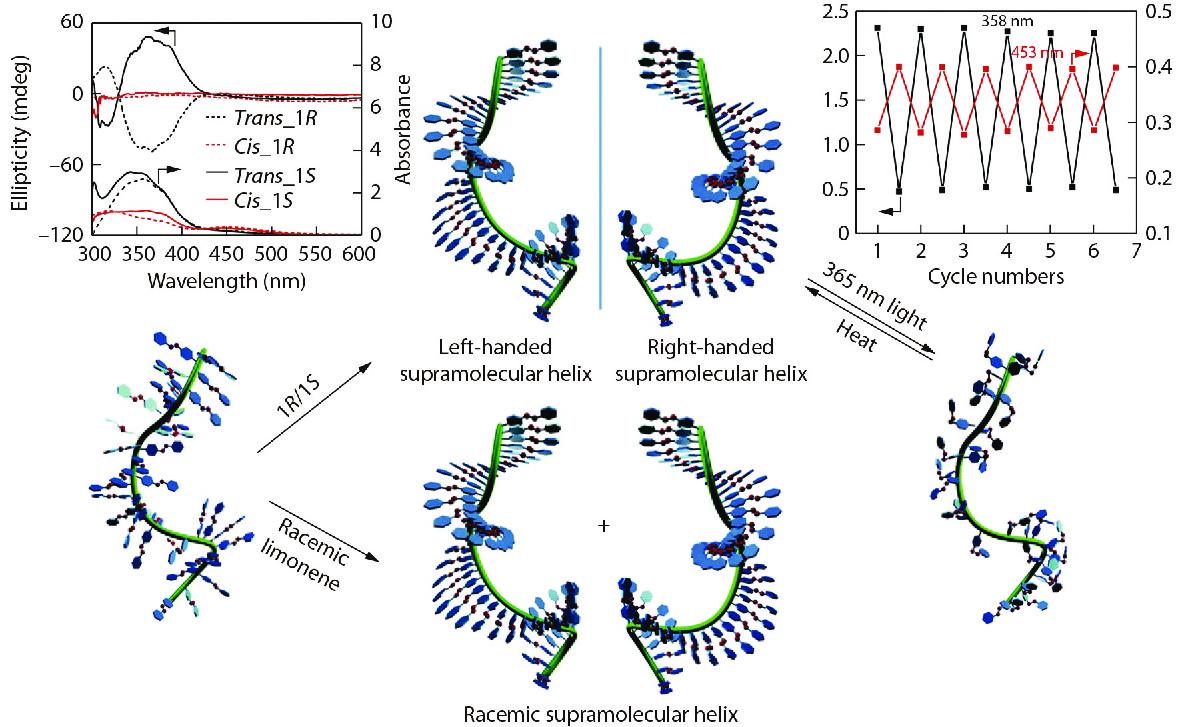
Chiral limonene is a commercially available, inexpensive and nontoxic asymmetric chiral source, which is attractive and widely used in green and cost-effective supramolecular assembly. Several examples constructing supramolecular chirality using chiral limonene have been presented in recent studies. For example, Fujiki et al. presented many pioneering studies on the limonene-induced chirality of π-conjugated polymers.[35−38] The optically active aggregates of π-conjugated homopolymer poly(9,9-di-n-dioctylfluorene) (PF8), poly(9,9-di-n-decylfluorene) (PF10) and its copolymers (F8T1 and F8T2 in Fig. 2) with CD and CPL dual signals can be quickly prepared in a ternary mixed solvent, chloroform (good solvent)/limonene (chiral solvent)/alkanol (poor solvent),[39] as shown in Fig. 2. The magnitude and sign of CD and CPL signals indicated that chiral properties of the aggregates were greatly influenced by the type of poor solvent and the enantiomeric purity of chiral limonene. In addition, the order of addition of limonene and methanol strongly affected the magnitude of the induced CD amplitude. The limonene-induction strategy provides a simple, easy and environmentally friendly approach for the rapid preparation of chiral polymer aggregates with special functions. Subsequently, Fujiki et al. proposed the possible inner interactions between chiral limonene molecules and achiral π-conjugated polymers to study the chirality transfer mechanism,[32,39] as presented in Fig. 3. Through the WAXD characterization of the aggregates formed with/without chiral limonene, it was found that the polymer aggregates were mainly formed by the π-π stacking of the conjugated backbone and the edge-to-edge stacking of the decyl chains. Without limonene, one major scattering peak (2θ=5.4°) corresponding to d-spacing of 16.3 Å was presented in wide-angle X-ray scattering spectra. This is assigned to the interchain distance of the PF10 chains with a spacer of decyl chains, as shown in Fig. 3. Unlike the more conventional hairy rod polymers, the side decyl chains in PF10 are perpendicular to the aromatic plane. Therefore, it was expected that the value of 16.3 Å would be much shorter than the sum of the two decyl chain lengths, which is estimated to be 25 Å, on the assumption that two decyl chains adopt the extended form normal to the fluorene rings. The observed d-spacing of 16.3 Å suggests that the n-decyl chains are significantly tilted relative to the direction of the edge-to-edge stacking of the PF10 main chains. However, the interchain distance of chiral PF10 aggregates after the induction of chiral limonene is 16.9 Å (2θ=5.2°), which is 0.6 Å longer than the value (16.3 Å) of PF10 aggregates without limonene. Meanwhile, the d-spacing of the π-π stacks remained unchanged during the induction process. These results suggest that the n-decyl chains are raised in the direction of the edge-to-edge stacking of the PF10 main chains due to the presence of limonene. Therefore, the limonene molecules likely exist closer to the n-decyl chains, leading to the incorporation of limonene in PF10 aggregates and resulting in the CH-π interaction between the chiral limonene and the decyl chains. Therefore, the backbone of achiral PF10 can undergo asymmetric helical stacking under the influence of chiral limonene environment during the assembly process. As a result, the chirality of chiral limonene molecules is transferred to achiral PF10 aggregates.
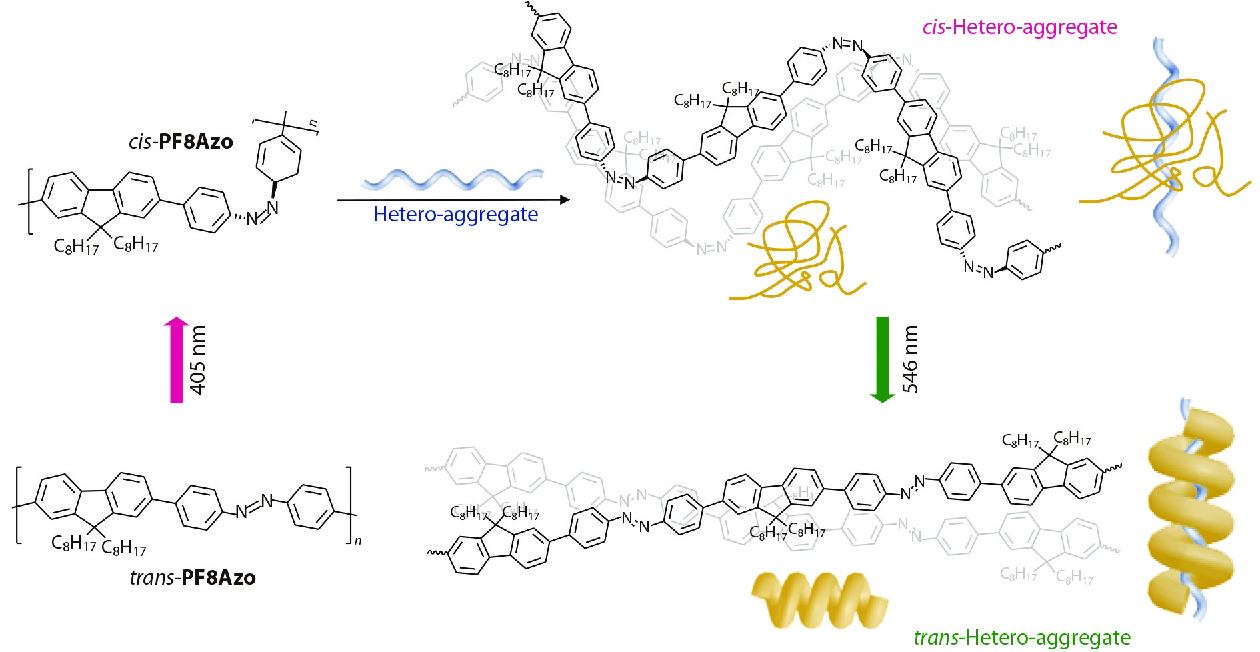
It is well known that azobenzene (Azo), as a photo-responsive chromophore, possesses reversible trans-cis photoisomerization properties.[40−44] Zhang and Fujiki et al. synthesized an Azo-containing π-conjugated polymer poly[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-4,4′-azobenzene] (PF8Azo) and studied its chiral supramolecular assembly behaviours induced by chiral limonene (Fig. 4).[45] The CD results showed that chirality was successfully transferred from S- or R-limonene solvent molecules to PF8Azo aggregates to generate optically active PF8Azo aggregates. Furthermore, reversible chiral switching was achieved by alternating light irradiation at 405 nm and 546 nm, due to the switching between the trans-origin aggregation and cis-origin disaggregation of PF8Azo in the mixed solvent.
The unique chemical structures of achiral polymers play an important role in the limonene-induced chirality transfer process. We first synthesized a class of achiral hyperbranched polymers (poly[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-bithiophene]s) with triphenylamine, triphenylbenzene and spirobifluorene as different branching units, and prepared a series of chiral and fluorescent nanoparticles using limonene as a chiral source.[46] In these systems, the structure and the content of different branching units have a significant effect on the induced chirality of achiral polymer particles, which provides powerful supplements to the understanding of chirality induction mechanism in π-conjugated polymer systems. Meanwhile, many other factors, such as the concentration of hyperbranched polymers, the type of poor solvent, and the ratio of chiral limonene also affected the chirality of achiral polymer particles. The same factors were also found to affect the chirality of achiral hyperbranched π-conjugated polymers-poly(9,9-di-n-octylfluorene)s (HPF8s) with hexaoctyltruxene (HT) as the branching unit (Fig. 4).[47] Furthermore, the element-dependent chiroptical inversion, as a novel phenomenon in polymer systems, was observed in achiral π-conjugated polymers in well-defined limonene induced chiral polymer particles.[48] Then achiral π-conjugated polymers, PF8, poly(9,9-di-n-octylsila-fluorenyl-2,7-diyl) (PSi8), poly(9-(1-octylnonyl)-9H-carbazole-2,7-diyl) (PCz8), and its copolymers (P(F8-alt-Si8), P(F8-alt-Cz8), and P(Si8-alt-Cz8) in Fig. 4), were also synthesized with C, N and Si atoms as the key factors to investigate the influence of atom structures (Fig. 4). The limonene chirality was efficiently transferred to PF8, PSi8 and P(F8-alt-PSi8) aggregates in mixed tersolvent, but not to PCz8, P(F8-alt-PCz8), and P(Si8-alt-PCz8) series. The chiroptical inactivity in Cz8-containing polymers could possibly be attributed to the stronger polarity of the polymers compared to chiral limonene, which results in weaker interactions between limonene and polymer. In addition, the induced chirality in PF8 and PSi8 was opposite under the same assembly conditions. Through chemical simulation and theoretical calculations, this can be ascribed to the opposite Mulliken charges between 9-Si in Si8 and 9-C in F8 units, which is −0.11 and +0.62, respectively. These studies provide a better understanding of chirality induction, transfer and inversion of π-conjugated polymers in aggregation states.
Generally, the tersolvent system, good solvent/limonene (chiral solvent)/poor solvent, is widely used in the chirality induction described above. However, a simplified solvent system will avoid the interactions between the building blocks and other solvent molecules. Further simplifying the assembled components of supramolecular system in achiral polymers can facilitate the study of chirality induction mechanism. Recently, our group designed the assembled solvent in achiral π-conjugated polymer systems using the chiral limonene as both the weak and the chiral solvents. Inspired from the special physicochemical properties of PF8 and the advantages of the simplest single solvent system, we used sol-gel strategy in neat limonene towards the production of PF8 assemblies.[49] The PF8 assemblies exhibited defined supramolecular chirality and fluorescence emissions, as shown in Fig. 5(a). The sol-gel process of PF8 was monitored by UV-Vis, CD, and fluorescent spectroscopies. Combined with self-assembly technology, the sol-gel transition was designed to produce supramolecular chirality in confined neat nonracemic limonene. In this system, supramolecular chirality of achiral PF8 was successfully induced by neat chiral limonene solvation and perfectly memorized for a long time, comparing favorably with that generated from chiral counterparts.[50] More importantly, the chiral assemblies of PF8 with obvious right- and left-handedness morphologies were clearly observed by AFM. In addition, the multilevel sensor for enantioselective recognition toward the limonene chirality was first achieved based on the achiral π-conjugated polymer, which may provide a new perspective for the design and construction of novel responsive materials.
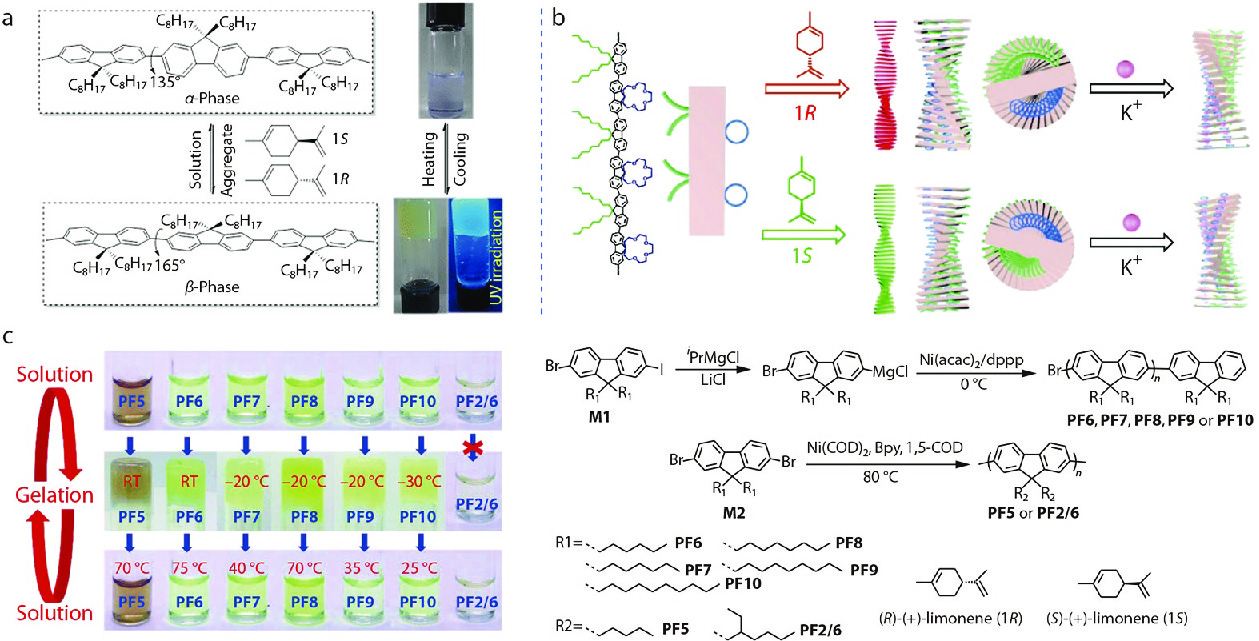
The achiral π-conjugated polymer structures can be flexibly designed to study the structure-property relationships in single neat chiral limonene. By manipulating the polymer structures, the stimulus-responsive supramolecular structures and its unique properties are facilely controlled in a single solvent system. For example, a novel optically inactive π-conjugated polyfluorene derivative (PF8C4, Fig. 5b) bearing the bulky crown ether pendant as the potassium ion-binding site was prepared using Suzuki coupling polymerization,[51] followed by the sol-gel transition process in a single solvent system as described above. The CD results indicated that polar crown ether group facilitated the supramolecular assembly of achiral polyfluorene to some extent by aggregating in neat chiral limonene. The corresponding P- and M-helical supramolecular structures were obtained and observed by TEM. Furthermore, upon appending K+ ions to the helical supramolecules, the optical activity of PF8C4 assemblies was further enhanced owing to the complexation between the K+ ions and crown ether pendants.
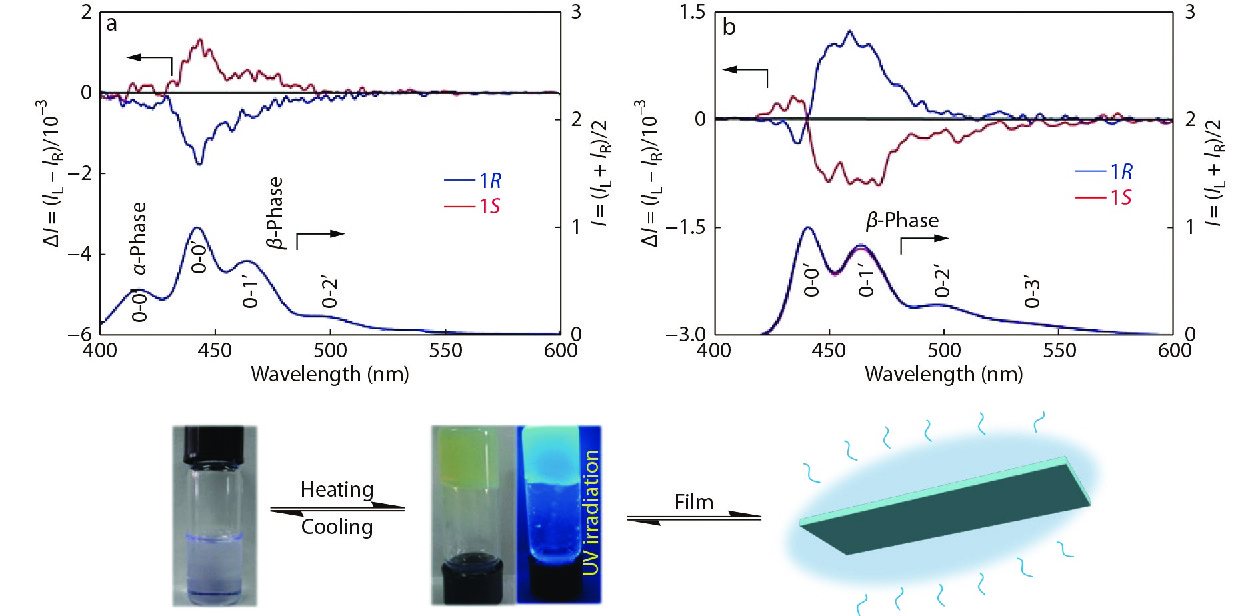
Recently, we reported another study about structures-property relationship where the parity of number of side chain carbons can affect the chirality induction of achiral polydialkylfluorenes (PFs) by chiral solvation.[52] A series of disubstituted PFs bearing linear or branched alkyl side-chains (PF5, PF6, PF7, PF8, PF9, PF10 and PF2/6 in Fig. 5) at the C9-positions of the fluorine moieties were synthesized. After sol-gel experiments, all PFs with linear side-chains can efficiently assemble into supramolecular chiral gels in neat limonene, but not PFs with branched side-chains (PF2/6) (Fig. 5c).[53] Meanwhile, combinatory analysis of CD, UV-Vis, and PL spectra confirmed the formation of β-phase conformers for PF5, PF7, PF8, PF9 and PF10, and another phase conformer for PF6. The results demonstrated that odd-numbered PFs (PF5, PF7, and PF9) formed β-phase conformation with a preferential helicity while the even-numbered PFs (PF8 and PF10) formed the opposite screw sense. Furthermore, the length and odd-even number of side-chains also influence the disassembling temperature for chiral PF gels. The results show that minor changes in the length of alkyl chains in achiral π-conjugated polymers can have a meaningful impact on its supramolecular assembly and chirality induction behavior, which can provide a reference for the design of chiral π-conjugated polymer and its potential applications, such as circularly polarized light emission, chiral sensors and chiroptical switch materials.[54−56]
Chiral solvation strategy has been well studied in achiral π-conjugated polymer systems as described above. However, the extension of this strategy to achiral side-chain polymer systems is rarely reported but very meaningful. Compared with π-conjugated polymers, achiral side-chain polymers have weaker inter-chain interactions and π-π stacking interactions. Therefore, it is more difficult for achiral side-chain polymers to form ordered supramolecular helical structures. To overcome this challenge, we currently propose that the photo-controllable solvation induction of supramolecular chirality can be achieved using achiral side-chain Azo-containing polymers. It is well known that Azo-polymers are promising reversible light-responsive materials due to their unique mechanical and physicochemical properties.[57−60] Generally, a coplanar trans-form Azo chromophore can be isomerized to bent-shaped cis-form by UV light irradiation and reversibly transformed back to its original trans-state by visible light or heat treatment, accompanied by changes in molecular configuration, polarity and other properties.[61] For example, Wang et al. reported the construction of shape-changeable colloidal spheres from amphiphilic Azo-polymers through gradual hydrophobic aggregation, which was achieved by adding the selective solvent into a completely dissolved solution of the polymer. These colloidal spheres showed interesting properties such as photoinduced shape deformation and photoinduced dichroism.[62−65] Yu et al. developed light-modulated actuators with Azo moieties as the light-responsive unit. Deformation and bending phenomenon of the crosslinked Azo-polymer films has been observed by irradiation under UV and visible light.[66−68] Furthermore, three-dimensional movements, such as a light-driven plastic motor, an inchworm walk, and a flexible robotic arm motion, of the crosslinked Azo-polymer films have been realized using light as the only stimulus.[69] These Azo-polymer materials can convert light energy directly into mechanical work without external forces, which is ascribed to the light-responsive properties caused by reversible trans-cis transition of the Azo units.
Meanwhile, the induction of chirality in achiral Azo-containing polymer system is very interesting and promising. Asymmetric π-π stacking of Azo building blocks may occur in the aggregation state due to the coplanar property of the trans-form. Therefore, supramolecular chirality induced by chiral solvation could be applied to Azo-containing superstructures. Recently, our group investigated the details of induction of supramolecular chirality in achiral side-chain Azo-polymers by chiral limonene solvation. We systematically designed and investigated a series of achiral side-chain Azo-polymers with different topological structures, such as linear,[70] cyclic[71] and star[72] polymers, where linear polymers were designed with different spacer lengths and push-pull electronic substituents (also shown in Fig. 4).[73] The results of CD and UV-Vis spectra suggested that the well-assembled supramolecular aggregates were successfully prepared in binary solvent and chirality was successfully transferred from nonracemic limonene to these achiral Azo-polymer aggregates. The supramolecular chirality obtained in aggregation can be ascribed to the ordered helical stacking of Azo building blocks on the side-chain through chiral limonene solvation (Fig. 6). Meanwhile, internal structures of Azo-polymers and external stimuli have distinctive influences on the induction and regulation of supramolecular chirality. This chirality can be modulated by UV light irradiation and heating-assisted reorganization process, which is due to the reversible trans-cis isomerization of the Azo unit. The reversible chiral-achiral switching based on achiral side-chain Azo-polymers was successfully achieved, which offers a new approach for the production of chiroptical materials. Furthermore, the preferred supramolecular chirality was successfully induced in assembled linear Azo polymers in film using solely neat limonene vapor.[74] The supramolecular chirality can be erased temporarily after heat treatment and recovered after cooling down to room temperature. A chiroptical switch can be easily achieved by alternately changing the temperature. The supramolecular chiral films also showed good chiral memory behavior for extended periods of time after removing the chiral sources.
Chiral Gelator
Low molecular weight organic molecules can immobilize solvents and self-assemble into ordered nanostructures, making supramolecular gelation a very simple strategy to construct nanostructures. Using an asymmetric gelator with a chiral factor, the strategy is further developed to achieve chirality transfer from the gelator to chiral assemblies via noncovalent interactions including π-π stacking, hydrogen bonding and donor-acceptor interactions.[75−77] The chiral gelation strategy, as a supramolecular cooperative assembly platform, provides a new approach for constructing well-defined chiral assemblies with supramolecular structures. Significant success in supramolecular systems has been achieved by Liu’s group by taking advantage of chiral gelators. For example, they designed and developed a universal chiral gelator, N,N′-bis(octadecyl)-L-Boc-glutamic diamide (LBG-18), that can co-gel most achiral organic compounds into supramolecular chiral assemblies.[78] The hydrophobic alkyl chains anchored on this gelator allows the chirality transfer from chiral gel matrix to achiral organic compounds. However, chirality induction and transfer by chiral gelation are usually limited between two small molecules.
Recently, our group and Prof. Liu mixed the same chiral gelator with achiral main-chain polymer PSi8, and further found that both the collaborative gelation and the chirality transfer from chiral gelators to achiral polymers occurred (Fig. 7a).[79] In this system, supramolecular chirality of polymers was obtained via the alkyl chain entanglements or interactions between the achiral polymer and gelator molecules. The helicity of the achiral polymer can be easily controlled by the molecular chirality of the gelator. Furthermore, the helicity of the supramolecular assemblies can be memorized even after the complete removal of gelator molecules. Importantly, CPL was emitted by the supramolecular assemblies because of the π-conjugated structures of fluorescent main-chain polymers, which originally inhere in the intrinsic chiral polymers. This work indicates that chiral gelation is a simple strategy to regulate achiral polymers into supramolecular chiral assemblies.
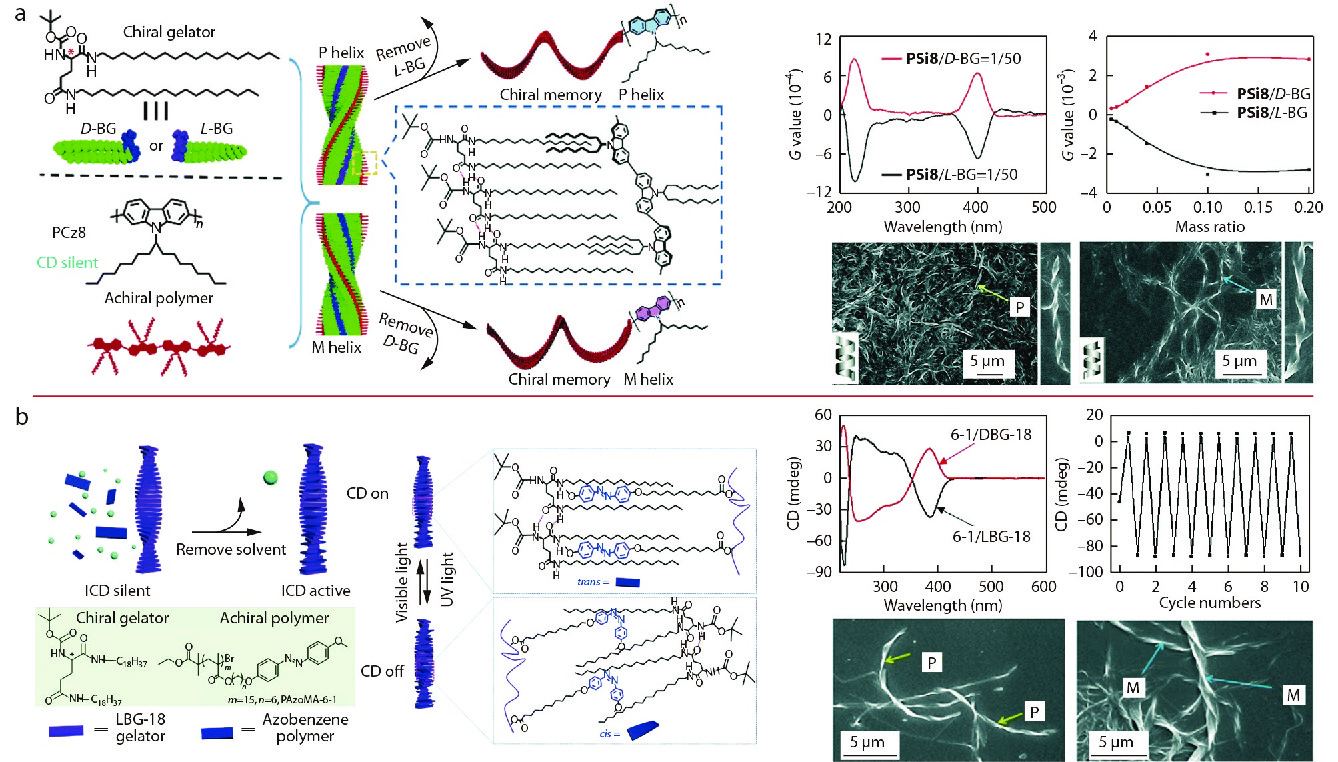
Inspired by this work, we further attempted to expand the application of chiral gelators to achiral side-chain Azo-polymers.[80] By manipulating the gelation of the mixed achiral side-chain Azo-polymers and chiral gelator, the supramolecular assemblies were easily and controllably constructed in situ with designable helical superstructures and supramolecular chirality (Fig. 7b). The induced CD of Azo-polymers can still be found in the xerogel state after complete removal of the cosolvent. Chiral field created by the assembled fibers of the mixed gel endowed the achiral Azo-polymer with helical conformation, which confirmed the successful chirality transfer from the gelator to the achiral polymer. Meanwhile, the constructed supramolecular chirality of the achiral Azo-polymers entirely followed the chirality of the gelator. Further study confirmed that the type of solvent and the length of alkyl chain play an important role in the construction of supramolecular chirality of achiral Azo-polymers. The supramolecular helical structures of achiral Azo-polymers cannot be induced by chiral gelators in the presence of the cosolvent, however, chirality induction and transfer processes occurred after the solvent is removed. Also, the gelator LBG-18 with longer alkyl chain is capable of transferring the chirality to achiral Azo-polymers but not LBGs with shorter alkyl chains. More importantly, a reversible chiroptical switch was successfully fabricated based on the reversible Azo trans-cis transition. These studies present a very accessible induction strategy guided by chiral gelator for achiral main-chain and side-chain polymers.
Circularly Polarized Light
CPL has been considered as one of the likely sources for the excess of chiral biological macromolecules as well as the single handed homochirality on earth and throughout the universe. The origins of the initial chirality may be ascribed to the asymmetric physical force from CPL during the star formation.[81] Furthermore, the circular and elliptical polarization phenomenon of sunlight has been observed in the nature due to the scattering of dusts and droplets. In the laboratory, the right-CPL (r-CPL) and left-CPL (l-CPL) can be easily obtained by using Glan-Taylor prism and 1/4 quartz waveplate (45o or 135o). Essentially, the circular polarization of light is the rotation of electric field wave in a plane perpendicular to its direction of propagation while maintaining a constant amplitude over time. Among the different methods for constructing supramolecular chirality, CPL is a massless and endless electromagnetic source. More importantly, CPL provides additional spatiotemporal control without limitation for the asymmetric properties of the polymers.
Actually, Nikolova’s group demonstrated the first example of CPL-induced chirality in achiral Azo-polymers. In 1997, they reported that when the achiral liquid crystalline Azo-polymer films were illuminated by one-handed CPL pumping, large circular birefringence and circular dichroism were clearly observed.[82] In addition, the sign of circular optical property of the achiral polymer films was determined by the handedness of CPL. Since then, CPL has become a well-accepted tool to induce the chirality of polymers. Further successful and extensive studies on the CPL-driven chirality induction of Azo-polymers were performed by Natansohn et al.,[83] Kim et al.,[84] Takezoe et al.,[85] Zou et al.[86] and Angiolini et al.[87] Furthermore, chirality has also been induced by using CPL, and experimentally and theoretically studied in several optically inactive polymers,[88] such as vinylpolymers, polydiacetylene, polyisocyanate and polyfluorene. For example, our group and Prof. Fujiki demonstrated for the first time CPL-modulated chiroptical generation, racemisation, inversion, switching and retention of μm-sized PF8T2 (Fig. 2) and PF8Azo (Fig. 4) polymer particles dispersed in organic solvents.[88,89] These chiral polymer structures could be effectively controlled by mixed solvents and CPL, using different variables such as the wavelength, irradiation time and ellipticity. In addition, Nakano et al. reported that achiral polyfluorenes can be optically active by creating macromolecular helical conformation in the backbone using CPL irradiation.[29,90] A mechanism involving a twisted-coplanar transition (TCT) of a fluorene-fluorene diad junction has been proposed. The basis of this theory is that the twisted and coplanar conformations are most stable in the ground state and in the excited state, respectively.[91] However, it should be noted that the chirality of these polyfluorene systems is attributed to the macromolecular helical structure of the polymer backbone, which is different from the supramolecular chirality of achiral polyfluorenes induced by chiral limonene.[49]
Most researchers concur that CPL handedness is the determining factor in the resulting polymeric chirality. Reversible helical switching of the one-handed structures in achiral polymers can be achieved by alternating the pumping of CPL with opposite handedness to the samples. However, the effect of CPL handedness, irradiation wavelength, and irradiation time on the chirality induction of polymers have not been systematically studied. The investigation of the dependence of photogeneration, photoresolution and photoswitching modes on the CPL wavelength is of great significance for studying the mechanism of chirality induction of achiral polymers.
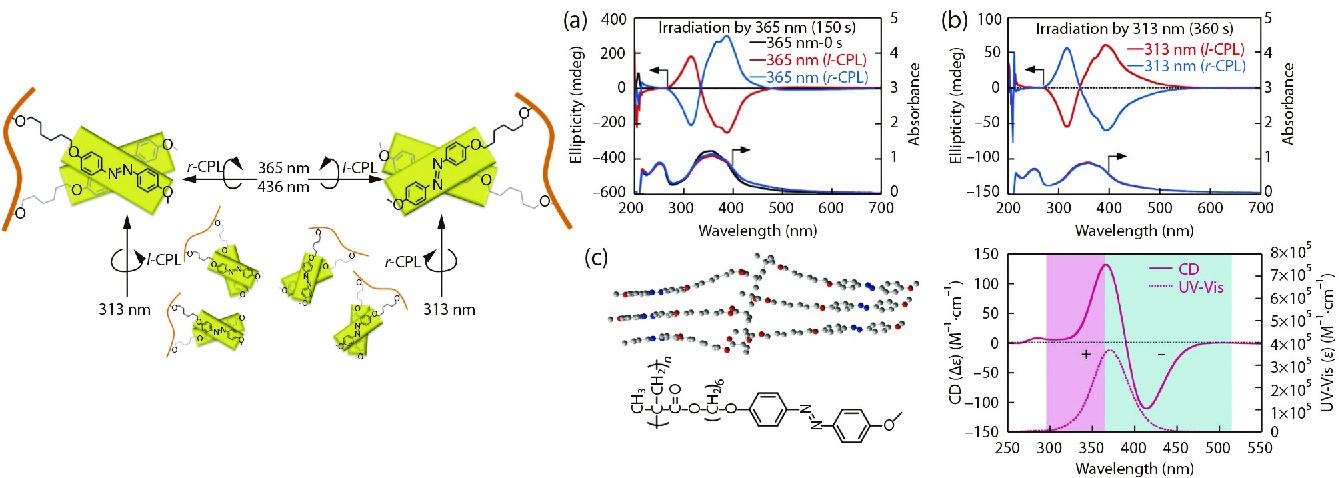
Recently, our group and Fujiki et al. demonstrated that the CPL wavelength (313 nm and 365/405/436 nm) is the determining factor for photogeneration, photoresolution and photoswitching of the achiral Azo-polymers, regardless of whether the handedness of the CPL source is the same.[92] As shown in Fig. 8(a), a completely symmetrical bisignate Cotton band at the π-π* transitions of Azo chromophores was observed as the intense exciton coupling effect in CD spectra upon l- and r-CPL (365 nm) irradiation. By irradiating with 365 nm l-CPL, a negative sign at the first Cotton band and a positive sign at the second Cotton band appeared. Interestingly, the CD signals were completely reversed when the Azo-polymers were irradiated by 313 nm l-CPL, and a positive sign at the first Cotton band and a negative sign at the second Cotton band were observed. (Fig. 8b). The results demonstrated that the supramolecular chiral structures with opposite handedness may be constructed in achiral Azo-polymers after irradiation with CPL of different wavelengths. Chirality induction, switching, memory and mechanism of chiroptical generation were also studied theoretically. Theoretical calculations and simulations with Zerner’s Intermediate Neglect of Differential Overlap (ZINDO) model were used to verify the origin of the bisignate Cotton bands of the induced chirality in achiral Azo-polymers (Fig. 8c). The results showed that the most favorable assorted structures of Azo building blocks tend to adopt chirally tilted π-π stacking motifs, which is believed to be responsible for the bisignate Cotton CD bands that were observed experimentally. In addition, the opposite Cotton bands at the long axis (~400 nm) and short axis (~300 nm) may be the origin of the dependence of chiroptical inversion on CPL handedness. These results describe a novel and efficient approach for the CPL-controlled chirality in achiral polymers which hold enormous potential in the mirror symmetry breaking field.
In addition to achiral side-chain polymers, Zhang and Fujiki et al. also demonstrated the first CPL-induced mirror symmetry breaking in achiral main-chain PF8Azo (Fig. 4) polymer particles dispersed in optofluidic organic solvents.[88] These chiroptical modes of generation, racemisation, inversion and retention could be effectively controlled by the molecular structure of the poor solvents (non-branched and branched alcohols) and the wavelength, pumping time and ellipticity of the CPL. Compared with other chirality inductions, CPL is a more efficient chiral physical source to be used in the supramolecular asymmetric assembly of achiral polymers due to its unique advantage of pure photochemical process, controllable physical quantities and lower chemical cost.
Chiral Dopant
The chiral doping strategy is a general method in chirality induction because chiral dopant can cooperatively self-assemble with achiral building blocks to form supramolecular assemblies.[93−95] For instance, Yu et al. systematically studied the hierarchical self-assembly process of Azo-containing liquid crystalline block copolymers doped with chiral tartaric acid.[21] Chirality from the chiral dopants was transferred to the supramolecular complex and induced the aggregation chirality of the achiral Azo block copolymers through hydrogen bonding between chiral tartaric acid, the poly(ethylene oxide) (PEO) segments and the Azo segments. Watkins et al. described the formation of helical phases in an achiral PEO-based block copolymer system by the straightforward use of chiral dopant driven self-assembly.[96] Hydrogen bonding interactions between chiral tartaric acid and PEO blocks not only enhance the phase segregation strength of the block copolymers but also transfer the chiral information from the dopant to the achiral backbone to induce the conformational chirality. To enrich chiral doping proposal, we prepared an optically active cofacial π-π phthalocyanine stacked supramolecular chiral nanofibers induced by equimolar amounts of inexpensive chiral diamine molecular sources.[97] Through acid-base interaction, achiral phthalocyanine can cooperatively self-assemble with chiral diamine molecules in an asymmetric manner. Specially, supramolecular chiral structures can be controlled by regulating the structures and molar ratio of the amine molecules, the volume ratio of poor/good cosolvents, the cavity metal of phthalocyanine and the addition order of the amines. Meanwhile, the chirality of nanofibers was persistent even after the addition of the opposite antipode diamine or the removal of pre-added chiral amine.
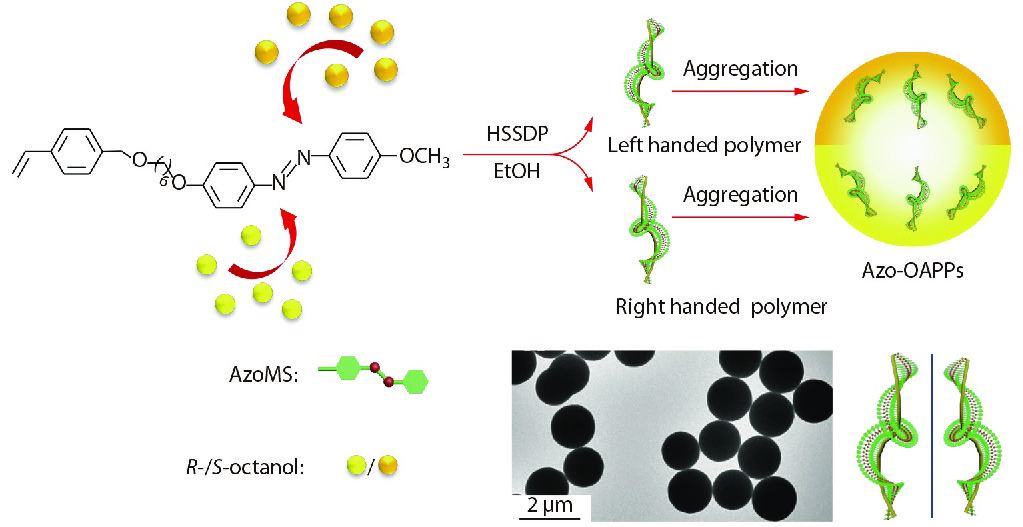
Recently, we have adopted chiral doping strategy during the polymerization process to prepare monodisperse Azo-containing micron-sized optically active polymer spheres with supramolecular chirality for the first time (Fig. 9).[98] By adding chiral octanol as an asymmetric source, achiral Azo-based monomers can self-assemble in situ into microspheres with supramolecular chirality in the side-chains during helix-sense-selective dispersion polymerization (HSSDP), which opens a new pathway for the controlled assembly of optically active polymer microspheres. The supramolecular chirality of polymer microspheres was verified to be derived from π-π stacking of achiral trans-Azo units in which the Azo units adopt a helical structure in the polymer side-chain. Interestingly, chirality of polymer microspheres can be perfectly memorized in dispersions for more than two months. Furthermore, an appropriate content of DVB crosslinker introduced into the Azo polymer chains did not destroy the supramolecular helical structures formed by the achiral side-chain trans-Azo units. Actually, the cross-linked network improved UV-radiation resistance of the optically active polymer spheres as compared with their non-crosslinked counterparts.
Chiral Scaffold/Template
In addition to chirality induction strategies described above, chiral biological and synthetic molecules can be used as scaffolds or templates to construct supramolecular chiral architectures from achiral or optically inactive polymers. The chiral scaffold/template induction has been proven to be a simple and effective method for producing supramolecular chirality from achiral substances. It should be noted that the chiral scaffold strategy is based on the non-covalent interactions between the host and the guest, which is a temporary platform for constructing a chiral composite structure. Thus chiral scaffold can be removed artificially after the induction process is completed, however, chiral template is generally difficult to be removed after chirality induction due to the strong interaction between the host and guest molecules. Therefore, chiral template is a long-lived platform.[99]
Many natural chiral/helical compounds can be used as chiral templates. For examples, schizophyllan adopts a right-handed triple helical structure in water but a single random coil conformation in dimethyl sulfoxide. Shinkai et al. reported that neutral one-dimensional schizophyllan can be used as hosts and chiral templates to induce the self-assembly of water-soluble polythiophene guests into supramolecular chiral insulated molecular wires.[100−102] Furthermore, cellulose, as the most abundant polysaccharide on earth, has been reported as a chiral scaffold capable of transferring its chirality to achiral/non-helical semi-flexible non-charged oligo- and poly(dialky-fluorene)s without other chiral catalysts.[103] Recently, it was found that a DNA-based origami supramolecular polymer can act as a chiral template to dynamically control the helical superstructure of gold nanorods.[104] The chirality induction of natural chiral templates has scientific significance in life sciences, especially in the study of the origin of chirality.
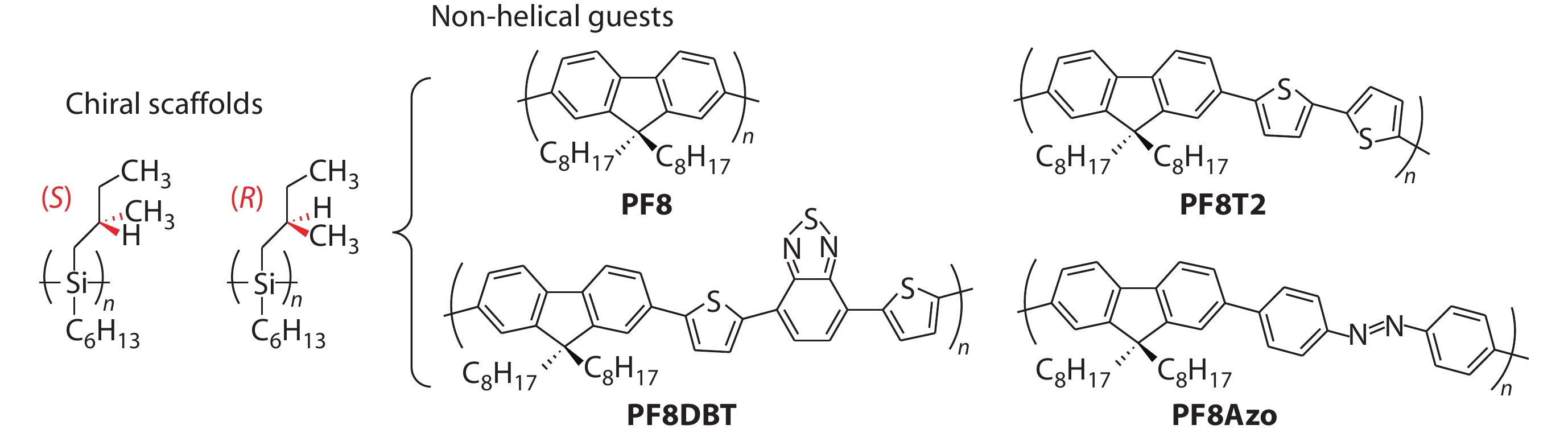
Chiral polysilane is an ideal photodegradable chiral scaffold for designing chiral scaffold-achiral polymer systems, due to its quick and complete decomposition upon 313 nm UV light irradiation. Fujiki et al. reported, for the first time, that CPL-active and CD-active achiral PF8, PF8DBT and PF8T2 (the structures are shown in Fig. 10) can be prepared through induction using photoscissorable helical scaffolds of non-charged poly(n-hexyl-(S)-2-methylbutylsiane) (PSi-S) and poly(n-hexyl-(R)-2-methylbutylsiane) (PSi-R).[105−107] At the same time, achiral polyfluorenes exhibits strong photochemical stability after the chirality induction process. After removing the chiral PSi scaffolds, the resulting π-conjugated polymers remain CPL- and CD-active. The significant difference in the photodegradability of chiral PSi and polyfluorenes is the key to designing the complex system. Recently, Zhang and Fujiki et al. effectively combined the scaffold-induced chirality and the photoisomerization of Azo units to construct a light-controlled reversible chiroptical switch.[108] The left and right helicities of PF8Azo (Fig. 10) aggregates were successfully controlled with an enantiomeric pair of rigid rod-like helical PSi-S and PSi-R, respectively. The chiroptical behavior of the hetero-aggregates was modulated by the trans-cis transformation of the photoresponsive Azo units (Fig. 11). Moreover, the optically active PF8Azo homo-aggregates were produced by complete photoscissoring of PSi at 313 nm, which could be assigned to the Siσ-Siσ* transitions of PSi-S and PSi-R.
APPLICATIONS OF INDUCED CHIRALITY
Supramolecular chirality has received much attention due to potential applications in chiral recognition and sensing, chiral switching, circularly polarized light, asymmetric catalysis and biological fields. For example, polyfluorenes have rigid planar structure, conferring higher optical properties, thermochemical stability, fluorescence quantum efficiency and wider energy level band gap.[109] Generally, polyfluorenes express CPL in both dissolved and aggregated state, which is usually derived from the intra-chain helical structures or inter-chain π-π helical stacking. Polyfluorenes with supramolecular chirality are one of the widely studied CPL materials. The CPL properties of polyfluorenes are influenced by their chemical structure, phase transition and supramolecular assembly behavior. In addition, supramolecular chirality can also be induced in achiral Azo-polymers through asymmetric stacking of Azo building blocks. The completely pure, by-product-free isomerization process has led to the rapid development of supramolecular chirality of Azo-polymers with a broad range of attractive applications.[110] Based on our results, we will summarize several applications of supramolecular chirality and provide an outlook on their prospects.
Chiral Recognition and Sensing
Chiral recognition and sensing are mainly based on the host-guest interactions, which have very important applications in supramolecular chemistry. Chiral recognition and sensing in supramolecular systems can reflect the interaction between supramolecular chiral assemblies and chiral molecules.[6] When a chiral host compound interacts with a pair of enantiomeric guests, the intermolecular chiral interaction between the host and each of the enantiomer is different, due to the different relative orientations of the interacting groups.[4] As a result, their different physical and chemical properties can be easily monitored by spectroscopic and other methods. Chiral recognition and sensing can be more easily achieved at the supramolecular level than that at molecular level. Furthermore, since supramolecular chirality can be constructed by chiral components, combinations of chiral and achiral components, or even completely achiral components, many achiral components can be used as chiral sensors to detect chirality.
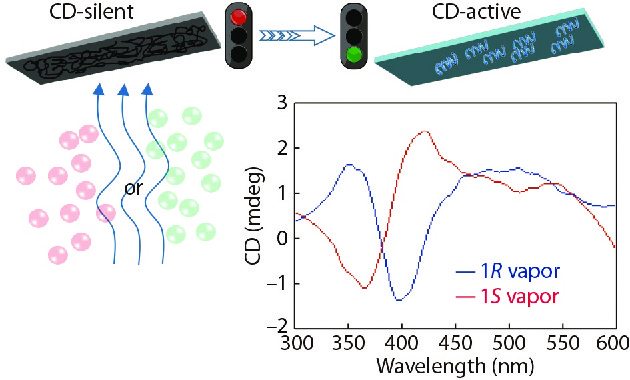
As described above, supramolecular chirality can be successfully constructed from achiral PF8 (Fig. 2) aggregates in nonracemic limonene by cooling PF8 solution at relatively low temperatures. The achiral PF8 film also has chiral sensing ability to chiral limonene vapors.[49] As presented in Fig. 12, achiral PF8 films were sensitive to chiral limonene vapors as shown by the corresponding peaks near 390 nm for both R-limonene (negative signal) and S-limonene (positive signal). This is the first example of chiral sensing film constructed using achiral π-conjugated polymers, presenting thermal stability as well as good optical properties. Furthermore, supramolecular chirality can also be constructed in the achiral side-chain Azo-polymer films through induction by limonene vapors.[74] Therefore, achiral Azo-polymer films can also be used as chiral sensors to detect the enantiomers of nonracemic limonene. These results will facilitate future research on the mechanism of chirality induction and transfer in achiral polymer systems and expand potential application in chiral film materials.
Chiroptical Switch
Since supramolecular chirality is usually constructed from chiral or achiral building blocks by weak noncovalent interactions, the coupling interaction between two building blocks is weaker and more changeable. Therefore, the dynamic supramolecular self-assembly process can be regulated by varying the temperature and the concentration of solutions, or the polarity of medium. The supramolecular chirality can be switched between “on” and “off” states or even inverted to each other under external stimuli by a reorganization process.[2] Supramolecular chirality constructed from achiral polymers are dynamic and can be easily controlled using external environment. This provides greater flexibility in the design of chiroptical switches.
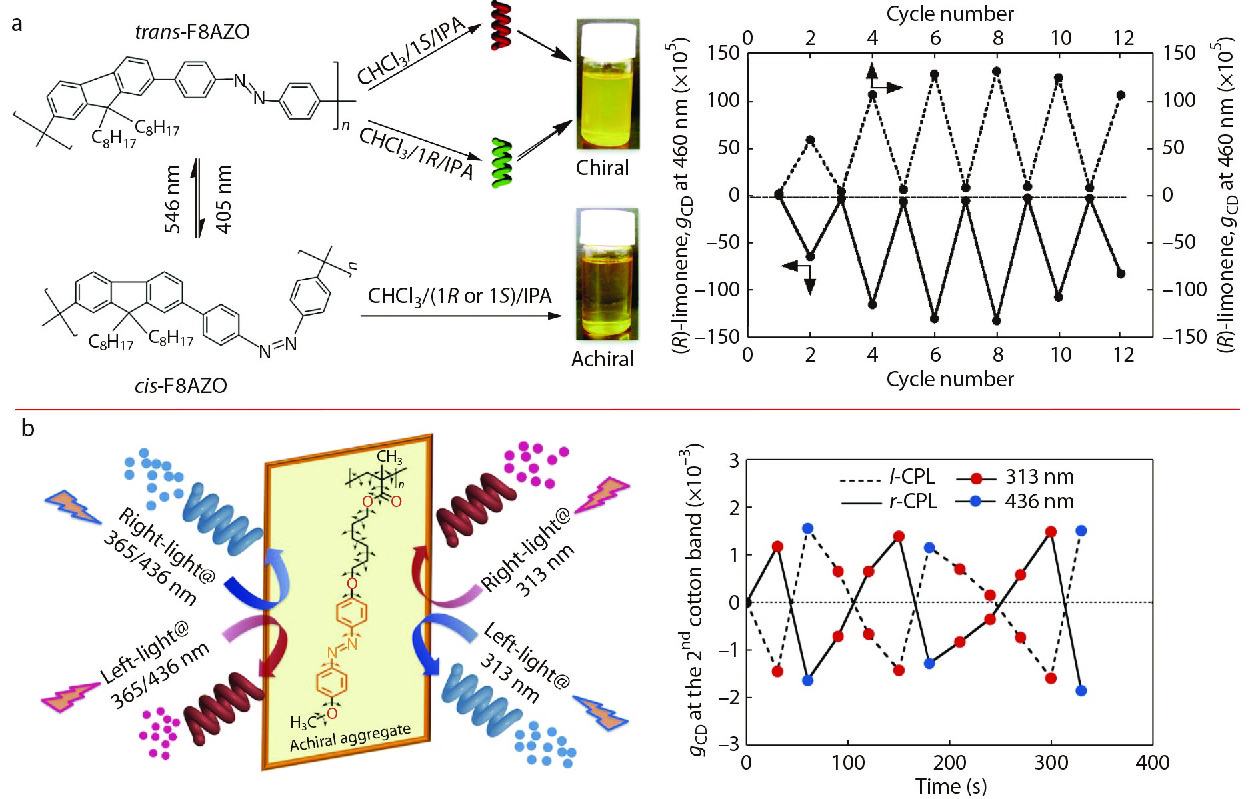
Liu et al. reported chiroptical switches composed of supramolecular assemblies that are formed using chiral gelators, which have been reviewed recently.[111] We also reported many examples of constructing chiroptical switches from achiral main-chain and side-chain polymers. Taking the achiral main-chain PF8Azo (Fig. 4) as an example, we reported that chirality of achiral PF8Azo aggregates can be induced by chiral limonene solvation.[45] This chirality can be erased by 405 nm light irradiation and recovered by 546 nm light irradiation. The results indicated that the aggregation-and-disaggregation characteristic of PF8Azo can be reversibly controlled by the trans-cis photoisomerizable Azo units in the presence of limonene molecules. This phenomenon originated from the switching between the trans-origin aggregation and cis-form disaggregation of PF8Azo in the mixed tersolvent. The reversible chiroptical switch can be repeated more than 5 times by alternating the wavelengths during photoirradiation (Fig. 13a). Chiroptical switches can also be constructed by achiral side-chain polymers, as seen in achiral Azo-polymer (Fig. 8) aggregates. We have demonstrated that a selected CPL can be used as a control for the construction of chiroptical switch, as demonstrated above.[92] A chiroptical switch of Azo-polymer aggregates in optofluidic medium can be constructed by two different wavelengths of CPL sources (r/l-CPL 313 nm and r/l-CPL 436 nm) with the same handedness. The CD value of the Azo-polymer aggregates was positive upon 313 nm r-CPL irradiation, and changed to negative upon 436 nm r-CPL irradiation. This dynamic process can be repeated many times, demonstrating the high resistance of the chiroptical switch (Fig. 13b).
Circularly Polarized Luminescence
Circularly polarized luminescence is a luminescence phenomenon that provides differential emission intensity of left and right circularly polarized light, thereby providing chirality and emission information on the excited state properties of the supramolecular chiral systems.[112] The differential emission of right and left circularly polarized light can be recorded by a spectroscopy instrument named circularly polarized luminescence spectrum. The circularly polarized luminescence spectrum reflects the differential emission; thus, it always accompanied with an emission spectrum.[113] In recent years, circularly polarized luminescence-active materials have received increasing attention due to their potential applications in sensors as well as display devices.[112] Owing to the development of supramolecular self-assembly, supramolecules have the potential to self-assemble into chiral assemblies with circularly polarized luminescence activity. Furthermore, the dissymmetry factor glum can be enhanced and easily controlled by adjusting the parameters of supramolecular self-assembly process. Through supramolecular self-assembly, our group has demonstrated that achiral polymers can be endowed with circularly polarized luminescence activity through chirality induction.
If the circularly polarized luminescence-sign or chiroptical switching and helicity of chiral luminescent materials in the photoexcited state can be effectively controlled, further insightful design and application of circularly polarized luminescence-active materials are possible. Actually, it is challenging to facilely and controllably prepare circularly polarized luminescence-active supramolecules with a high dissymmetry ratio, glum, from entirely achiral polymer systems. We found that PF8 supramolecular aggregates in neat limonene solvents revealed relatively intense, vibronic circularly polarized luminescence bands due to the formation of β-phase.[49] Besides, apparent circularly polarized luminescence signs from PF8 film were opposite to those from supramolecular aggregates in gel state, despite the identical chirality of the limonene used (Fig. 14). Furthermore, an exciton couplet bisignate signals appeared in circularly polarized luminescence spectra, indicating the possibility of photoexcited state chirality. We assumed that circularly polarized luminescence-sign inversion is a common characteristic, that depends on homogeneity of the solution, gel, and condensed film states. These results may provide a new way for designing and controlling circularly polarized luminescence-active π-conjugated polymer materials.
CHALLENGES AND OUTLOOK
A number of excellent research studies and remarkable achievements have been made in supramolecular chiral chemistry. However, there are still existing challenges and opportunities in this field:
(1) The construction of supramolecular chirality induced by chiral solvent described above requires first the synthesis of the corresponding polymers, followed by self-assembly under selective conditions, regardless of achiral main-chain π-conjugated polymers or side-chain Azo-polymers. This means that the construction of supramolecular chiral structures require at least two steps: synthesizing the corresponding polymers followed by performing the assembly in an asymmetric solvent environment. Furthermore, many supramolecular chiral aggregates prepared by this method are performed at a very low solid content (even below 0.1 wt%), which have poor stability and are easy to precipitate in the mixed solvent systems. Hence it is difficult to prepare them in large quantities. If there is a one-step strategy to construct the supramolecular chiral assembly during polymerization would allow the process to be simpler and more economical.
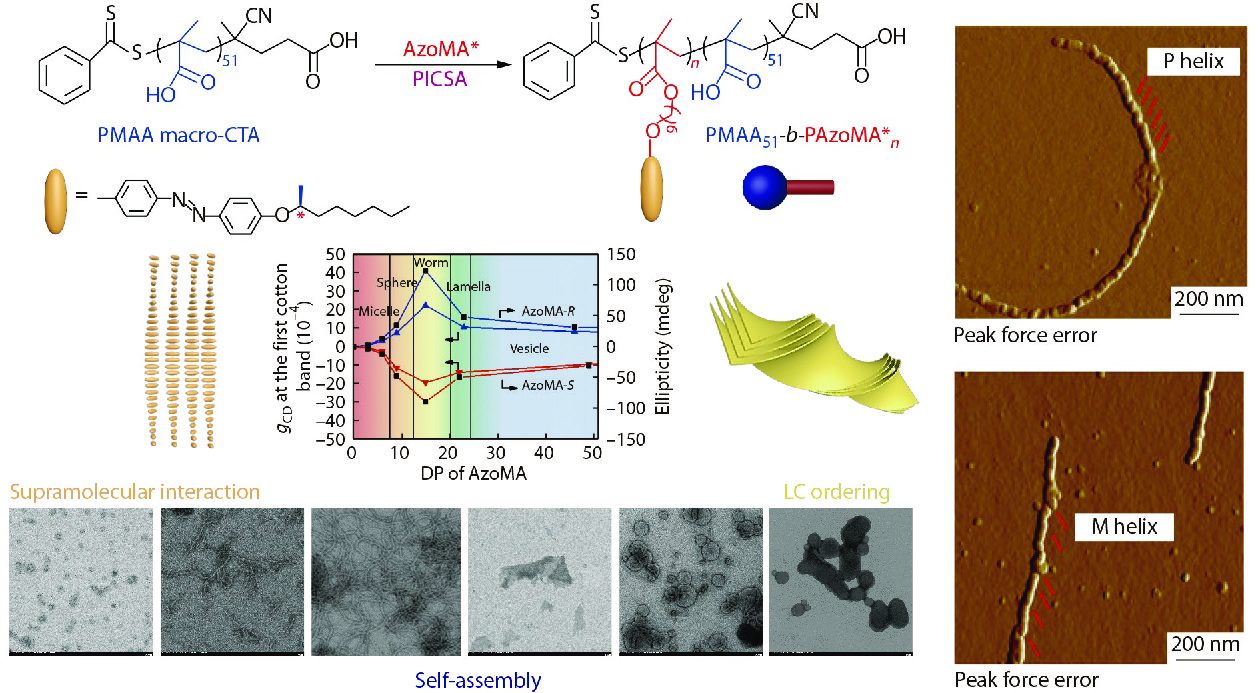
Recently, we demonstrated for the first time the polymerization-induced chiral self-assembly (PICSA) strategy,[114] as presented in Fig. 15. Hierarchical supramolecular chiral Azo-polymer assemblies were obtained at a high solid content (10 wt%) using PISA strategy in association with reversible addition-fragmentation chain transfer (RAFT)-mediated dispersion polymerization. PICSA allows the controlled self-assembly of Azo-polymers in a single solvent during polymerization with simultaneous liquid crystalline ordering, supramolecular interaction and self-assembly. During PICSA process, solvophilic poly(methacrylic acid) macromolecular chain transfer agent (PMAA macro-CTA) is used to mediate the polymerization of soluble monomer in a selective solvent. As the polymerization proceeds, the solubility of Azo polymer chains decreases, driving the in situ self-assembly of amphiphilic Azo-polymers into different morphological phases, such as spheres, worms, fibers, lamellae and vesicles. Modulating solvophobic chiral side-chain Azo blocks, supramolecular chiral assemblies can be obtained in a controlled manner with helical stacking taking place spontaneously in the Azo semectic layers. Furthermore, the supramolecular chiral assembly becomes more stable due to its amphiphilic nature. More interestingly, the morphological transition of Azo-polymer assemblies has a significant effect on the induction and expression of supramolecular chirality, and the assemblies with fibrillar morphology have the most helical ordered superstructure compared with other counterparts. In addition to the side-chain Azo-polymer assemblies, Wan et al.[115] and O’Reilly et al.[116] recently reported that PICSA strategy can also be employed to prepare main-chain helical assemblies, such as poly(phenylacetylene)s and poly(aryl isocyanide)s. Compared with traditional methods for preparation of these supramolecular chiral assemblies, PICSA strategy can effectively construct supramolecular assemblies at high concentration with controlled hierarchical morphologies and further functionalize materials with manipulatable chirality.
(2) In the induction method described above, the indispensable role of chiral sources in the process of supramolecular assembly, and even how many traces of chiral sources actually work, still remain unclear in chirality induction process. In the future, through rational structural design of the targeted achiral polymers, a chiral seed may be covalently linked to achiral polymer chains, for example at a fixed position in the polymer backbone, to control the cooperative assembly as well as supramolecular chiral superstructures. This method will overcome the inherent dependence of the traditional chirality induction methods on external chiral sources, such as chiral solvents, chiral gelators and circularly polarized light, etc.
(3) Currently, supramolecular chirality constructed in achiral polymer systems is mainly based on the weak noncovalent interactions, which is easily destroyed by external stimuli, such as light, heat or solvent. The destroyed chiral structure cannot be recovered without the pre-chiral source. This shortcoming greatly limits their applications in new functional materials. Therefore, precise modulation of the network topologies and mechanical properties in achiral polymers may further expand the potential applications of supramolecular chirality. For example, we are managing to design a micro-system from the cross-linking achiral polymers in which the induced chirality of the nanostructure will be stored permanently and capable of self-recovery when exposed to the external stimulus. This methodology will open a new route towards the formation of chiral self-recovery based materials from absolutely achiral polymers.
(4) In nature, molecular-scale motion can power macroscopic mechanical motion in highly complex processes. Biological systems based on helical motion are common, such as coiled tendrils and snail's shell. The supramolecular chiral superstructures constructed by chirality induction in achiral polymers are usually microscopic. Based on the general concept that such microscopic helical deformations may similarly occur in artificial systems, the construction of a macroscopic helix to mimic the chiral bias in nature is an important topic to be further explored. This means that molecular chirality would be translated across microscopic scale lengths into macroscopic helix, which is of great significance for the construction of chiral functional materials.
CONCLUSIONS
In summary, we have mainly summarized the progress of our research work and others on the construction of supramolecular chirality in polymer systems based on the chirality induction, transfer as well as the applications in different fields. We have provided many examples of the chirality induction, regulation and unique features of supramolecular chirality in achiral polymer systems using chiral solvents, chiral gelators, circularly polarized light, chiral dopants and chiral scaffolds/templates. Besides the chirality induction in achiral polymer systems, we also reported another highly efficient strategy, the polymerization induced chiral self-assembly (PICSA), for constructing hierarchical supramolecular chiral superstructures in situ. The details and advantages of PICSA can be referred to previous research[114] and review.[2] In addition, some novel applications, such as chiral recognition and sensing, chiroptical switching, and circularly polarized luminescence, have also been investigated. Remarkable developments have been made on the construction of supramolecular chirality in polymer systems, as represented by diverse strategies available, however, the further exploration and application of supramolecular chirality still face some great challenges. Although there are many uncertainties and difficulties in the fields, “all things before maturity have a bitter taste”. We hope that this feature article will provide new insights into the investigation of mechanism and structure design, and further advance the development of supramolecular chiral chemistry.
BIOGRAPHY
Wei Zhang received his Ph.D. degree from Soochow University in 2006. He joined the College of Chemistry, Chemical Engineering and Materials Science, Soochow University in 2006 and was appointed as an Associate Professor at Soochow University in 2009. He did postdoctoral research (JSPS Fellow, 2009−2011) at the Nara Institute of Science and Technology (NAIST) with Prof. Michiya Fujiki. He was promoted as a Full Professor in 2013. He did research as Visiting Professor (2015. 11−2016. 02) in Hokkaido University in Prof. Tamaki Nakano’s group. He leads a polymer chemistry group working on chiral polymer synthesis, chiral assembly, and responsive polymer materials.
Helical polymers: Synthesis, structures, and functions
Chem. Rev. 2009 109 6102 6211Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102−6211.
Supramolecular chirality in azobenzene-containing polymer system: traditional postpolymerization self-assembly versus in situ supramolecular self-assembly strategy
Int. J. Mol. Sci. 2020 21 6186Cheng, X. X.; Miao, T. F.; Qian, Y. L.; Zhang, Z. B.; Zhang, W.; Zhu, X. L. Supramolecular chirality in azobenzene-containing polymer system: traditional postpolymerization self-assembly versus in situ supramolecular self-assembly strategy. Int. J. Mol. Sci. 2020, 21, 6186.
Pasteur and the art of chirality
Nat. Chem. 2017 9 604 605Gal, J. Pasteur and the art of chirality. Nat. Chem. 2017, 9, 604−605.
Chirality-sensing supramolecular systems
Chem. Rev. 2008 108 1 73Hembury, G. A.; Borovkov, V. V.; Inoue, Y. Chirality-sensing supramolecular systems. Chem. Rev. 2008, 108, 1−73.
Chirality-responsive helical polymers
Macromolecules 2008 41 3 12Yashima, E.; Maeda, K. Chirality-responsive helical polymers. Macromolecules 2008, 41, 3−12.
Supramolecular chirality in self-assembled systems
Chem. Rev. 2015 115 7304 7397Liu, M. H.; Zhang, L.; Wang, T. Y. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304−7397.
Analysis of stereoisomers of chiral drug by mass spectrometry
Chirality 2018 30 609 618Chen, X. L.; Kang, Y.; Zeng, S. Analysis of stereoisomers of chiral drug by mass spectrometry. Chirality 2018, 30, 609−618.
Controlling supramolecular chirality in multicomponent self-assembled systems
Acc. Chem. Res. 2018 51 2324 2334Xing, P. Y.; Zhao, Y. L. Controlling supramolecular chirality in multicomponent self-assembled systems. Acc. Chem. Res. 2018, 51, 2324−2334.
Helical crystal assemblies in nonracemic chiral liquid crystalline polymers: where chemistry and physics meet
Ind. Eng. Chem. Res. 2010 49 11936 11947Wang, J.; Li, C. Y.; Jin, S.; Weng, X.; Van Horn, R. M.; Graham, M. J.; Zhang, W. B.; Jeong, K. U.; Harris, F. W.; Lotz, B.; Cheng, S. Z. D. Helical crystal assemblies in nonracemic chiral liquid crystalline polymers: where chemistry and physics meet. Ind. Eng. Chem. Res. 2010, 49, 11936−11947.
Supramolecular chirality of self-assembled systems in solution
Chem. Soc. Rev. 2004 33 363 372Mateos-Timoneda, M. A.; Crego-Calama, M.; Reinhoudt, D. N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004, 33, 363−372.
Programmed high-hole-mobility supramolecular polymers from disk-shaped molecules
Adv. Funct. Mater. 2010 20 3941 3947Zhang, W.; Ochi, K.; Fujiki, M.; Naito, M.; Ishikawa, M.; Kaneto, K.; Takashima, W.; Saeki, A.; Seki, S. Programmed high-hole-mobility supramolecular polymers from disk-shaped molecules. Adv. Funct. Mater. 2010, 20, 3941−3947.
Circularly polarized blue luminescent spherulites consisting of hierarchically assembled Ionic conjugated polymers with a helically π-stacked structure
Adv. Mater. 2012 24 6451 6456Watanabe, K.; Iida, H.; Akagi, K. Circularly polarized blue luminescent spherulites consisting of hierarchically assembled Ionic conjugated polymers with a helically π-stacked structure. Adv. Mater. 2012, 24, 6451−6456.
Chiral helicity induced by hydrogen bonding and chirality of podand histidyl moieties
Org. Lett. 2001 3 1459 1461Moriuchi, T.; Nishiyama, M.; Yoshida, K.; Ishikawa, T.; Hirao, T. Chiral helicity induced by hydrogen bonding and chirality of podand histidyl moieties. Org. Lett. 2001, 3, 1459−1461.
Molecular description of the propagation of chirality from molecules to complex systems: different mechanisms controlled by hydrophobic interactions
Chem. Eur. J. 2012 18 14680 14688Marinelli, F.; Sorrenti, A.; Corvaglia, V.; Leone, V.; Mancini, G. Molecular description of the propagation of chirality from molecules to complex systems: different mechanisms controlled by hydrophobic interactions. Chem. Eur. J. 2012, 18, 14680−14688.
Differential self-assembly and tunable emission of aromatic peptide bola-amphiphiles containing perylene bisimide in polar solvents including water
Langmuir 2014 30 7576 7584Bai, S.; Debnath, S.; Javid, N.; Frederix, P. W. J. M.; Fleming, S.; Pappas, C.; Ulijn, R. V. Differential self-assembly and tunable emission of aromatic peptide bola-amphiphiles containing perylene bisimide in polar solvents including water. Langmuir 2014, 30, 7576−7584.
Dynamic modulation of supramolecular chirality driven by factors from internal to external levels
Chem. Asian J. 2019 14 2172 2180Yue, B. B.; Zhu, L. L. Dynamic modulation of supramolecular chirality driven by factors from internal to external levels. Chem. Asian J. 2019, 14, 2172−2180.
Helical conformations of poly(3,5-disubstituted phenylacetylene)s tuned by pendant structure and solvent
Macromolecules 2017 50 3489 3499Wang, S.; Feng, X. Y.; Zhang, J.; Yu, P.; Guo, Z. X.; Li, Z. B.; Wan, X. H. Helical conformations of poly(3,5-disubstituted phenylacetylene)s tuned by pendant structure and solvent. Macromolecules 2017, 50, 3489−3499.
Expression of supramolecular chirality in block copoly(thiophene)s
Macromolecules 2010 43 3794 3800Van den Bergh, K.; Cosemans, I.; Verbiest, T.; Koeckelberghs, G. Expression of supramolecular chirality in block copoly(thiophene)s. Macromolecules 2010, 43, 3794−3800.
Solvent-dependent switch of helical main-chain chirality in sergeants-and-soldiers-type poly(quinoxaline-2,3-diyl)s: effect of the position and structures of the "sergeant" chiral units on the screw-sense induction
J. Am. Chem. Soc. 2013 135 10104 10113Nagata, Y.; Yamada, T.; Adachi, T.; Akai, Y.; Yamamoto, T.; Suginome, M. Solvent-dependent switch of helical main-chain chirality in sergeants-and-soldiers-type poly(quinoxaline-2,3-diyl)s: effect of the position and structures of the "sergeant" chiral units on the screw-sense induction. J. Am. Chem. Soc. 2013, 135, 10104−10113.
Photoinduced chiral nematic organization in an achiral glassy nematic azopolymer
Adv. Funct. Mater. 2007 17 3486 3492Tejedor, R. M.; Oriol, L.; Serrano, J. L.; Urena, F. P.; Gonzalez, J. J. L. Photoinduced chiral nematic organization in an achiral glassy nematic azopolymer. Adv. Funct. Mater. 2007, 17, 3486−3492.
Hierarchical self-assembly in liquid-crystalline block copolymers enabled by chirality transfer
Angew. Chem. Int. Ed. 2018 57 12524 12528Huang, S.; Chen, Y. X.; Ma, S. D.; Yu, H. F. Hierarchical self-assembly in liquid-crystalline block copolymers enabled by chirality transfer. Angew. Chem. Int. Ed. 2018, 57, 12524−12528.
Helical polyacetylene: asymmetric polymerization in a chiral liquid-crystal field
Chem. Rev. 2009 109 5354 5401Akagi, K. Helical polyacetylene: asymmetric polymerization in a chiral liquid-crystal field. Chem. Rev. 2009, 109, 5354−5401.
Circularly polarized luminescence from supramolecular chiral complexes of achiral conjugated polymers and a neutral polysaccharide
Chem. Lett. 2009 38 254 255Haraguchi, S.; Numata, M.; Li, C.; Nakano, Y.; Fujiki, M.; Shinkai, S. Circularly polarized luminescence from supramolecular chiral complexes of achiral conjugated polymers and a neutral polysaccharide. Chem. Lett. 2009, 38, 254−255.
Poly((4-carboxyphenyl)acetylene) as a probe for chirality assignment of amines by circular-dichroism
J. Am. Chem. Soc. 1995 117 11596 11597Yashima, E.; Matsushima, T.; Okamoto, Y. Poly((4-carboxyphenyl)acetylene) as a probe for chirality assignment of amines by circular-dichroism. J. Am. Chem. Soc. 1995, 117, 11596−11597.
Transfer and amplification of chiral molecular information to polysilylene aggregates
J. Am. Chem. Soc. 2001 123 4847 4848Nakashima, H.; Koe, J. R.; Torimitsu, K.; Fujiki, M. Transfer and amplification of chiral molecular information to polysilylene aggregates. J. Am. Chem. Soc. 2001, 123, 4847−4848.
Transformation from preformed racemic helical poly(phenylacetylene)s to the enantioenriched helical polymers by chiral solvation, followed by removal of the chiral solvents
Polym. J. 2012 44 327 333Kaneko, T.; Liang, X. Y.; Kawami, A.; Sato, M.; Namikoshi, T.; Teraguchi, M.; Aoki, T. Transformation from preformed racemic helical poly(phenylacetylene)s to the enantioenriched helical polymers by chiral solvation, followed by removal of the chiral solvents. Polym. J. 2012, 44, 327−333.
Helical polyaniline nanofibers induced by chiral dopants by a polymerization process
Adv. Mater. 2007 19 3353 3357Yan, Y.; Yu, Z.; Huang, Y. W.; Yuan, W. X.; Wei, Z. X. Helical polyaniline nanofibers induced by chiral dopants by a polymerization process. Adv. Mater. 2007, 19, 3353−3357.
Stimuli-responsive helical poly(phenylacetylene)s bearing cyclodextrin pendants that exhibit enantioselective gelation in response to chirality of a chiral amine and hierarchical super-structured helix formation
Macromolecules 2011 44 3217 3226Maeda, K.; Mochizuki, H.; Osato, K.; Yashima, E. Stimuli-responsive helical poly(phenylacetylene)s bearing cyclodextrin pendants that exhibit enantioselective gelation in response to chirality of a chiral amine and hierarchical super-structured helix formation. Macromolecules 2011, 44, 3217−3226.
Molecular chirality induction to an achiral π-conjugated polymer by circularly polarized light
Chem. Commun. 2012 48 1871 1873Wang, Y.; Sakamoto, T.; Nakano, T. Molecular chirality induction to an achiral π-conjugated polymer by circularly polarized light. Chem. Commun. 2012, 48, 1871−1873.
The macromolecular route to chiral amplification
Angew. Chem. Int. Ed. 1999 38 3139 3154Green, M. M.; Park, J. W.; Sato, T.; Teramoto, A.; Lifson, S.; Selinger, R. L. B.; Selinger, J. V. The macromolecular route to chiral amplification. Angew. Chem. Int. Ed. 1999, 38, 3139−3154.
Induction of preferential helical screw senses in optically inactive polysilanes via chiral solvation
Macromol. Rapid Commun. 2002 23 99 103Dellaportas, P.; Jones, R. G.; Holder, S. J. Induction of preferential helical screw senses in optically inactive polysilanes via chiral solvation. Macromol. Rapid Commun. 2002, 23, 99−103.
Ambidextrous circular dichroism and circularly polarised luminescence from poly(9,9-di-n-decylfluorene) by terpene chirality transfer
Polym. Chem. 2010 1 460 469Nakano, Y.; Liu, Y.; Fujiki, M. Ambidextrous circular dichroism and circularly polarised luminescence from poly(9,9-di-n-decylfluorene) by terpene chirality transfer. Polym. Chem. 2010, 1, 460−469.
Optically active conjugated polymer nanoparticles from chiral solvent annealing and nanoprecipitation
Macromolecules 2015 48 4754 4757Kim, H.; Jin, Y. J.; Kim, B. S. I.; Aoki, T.; Kwak, G. Optically active conjugated polymer nanoparticles from chiral solvent annealing and nanoprecipitation. Macromolecules 2015, 48, 4754−4757.
A macromolecular conformational change driven by a minute chiral solvation energy
J. Am. Chem. Soc. 1993 115 4941 4942Green, M. M.; Khatri, C.; Peterson, N. C. A macromolecular conformational change driven by a minute chiral solvation energy. J. Am. Chem. Soc. 1993, 115, 4941−4942.
Solvent-to-polymer chirality transfer in intramolecular stack structure
Macromolecules 2012 45 5379 5386Lee, D.; Jin, Y. J.; Kim, H.; Suzuki, N.; Fujiki, M.; Sakaguchi, T.; Kim, S. K.; Lee, W. E.; Kwak, G. Solvent-to-polymer chirality transfer in intramolecular stack structure. Macromolecules 2012, 45, 5379−5386.
Chiral optofluidics: gigantic circularly polarized light enhancement of all-trans-poly(9,9-di-n-octylfluorene-2,7-vinylene) during mirror-symmetry-breaking aggregation by optically tuning fluidic media
RSC Adv. 2012 2 6663 6671Fujiki, M.; Jalilah, A.; Suzuki, N.; Taguchi, M.; Zhang, W.; Abdellatif, M. M.; Nomura, K. Chiral optofluidics: gigantic circularly polarized light enhancement of all-trans-poly(9,9-di-n-octylfluorene-2,7-vinylene) during mirror-symmetry-breaking aggregation by optically tuning fluidic media. RSC Adv. 2012, 2, 6663−6671.
Mirror-symmetry-breaking in poly[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-biphenyl] (PF8P2) is susceptible to terpene chirality, achiral solvents, and mechanical stirring
Molecules 2013 18 7035 7057Fujiki, M.; Kawagoe, Y.; Nakano, Y.; Nakao, A. Mirror-symmetry-breaking in poly[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-biphenyl] (PF8P2) is susceptible to terpene chirality, achiral solvents, and mechanical stirring. Molecules 2013, 18, 7035−7057.
Chiroptical generation and inversion during the mirror-symmetry-breaking aggregation of dialkylpolysilanes due to limonene chirality
Chem. Commun. 2012 48 6636 6638Nakano, Y.; Ichiyanagi, F.; Naito, M.; Yang, Y. G.; Fujiki, M. Chiroptical generation and inversion during the mirror-symmetry-breaking aggregation of dialkylpolysilanes due to limonene chirality. Chem. Commun. 2012, 48, 6636−6638.
Limonene magic: Noncovalent molecular chirality transfer leading to ambidextrous circularly polarised luminescent π-conjugated polymers
New J. Chem. 2010 34 637 647Kawagoe, Y.; Fujiki, M.; Nakano, Y. Limonene magic: Noncovalent molecular chirality transfer leading to ambidextrous circularly polarised luminescent π-conjugated polymers. New J. Chem. 2010, 34, 637−647.
Structural design and application of azo-based supramolecular polymer systems
Chinese J. Polym. Sci. 2019 37 1183 1199Yu, H. T.; Tang, J. W.; Feng, Y. Y.; Feng, W. Structural design and application of azo-based supramolecular polymer systems. Chinese J. Polym. Sci. 2019, 37, 1183−1199.
Azobenzene based photo-responsive mechanical actuator fabricated by intermolecular H-bond interaction
Chinese J. Polym. Sci. 2021 39 417 424Yu, C. Y.; Mu, J. H.; Fu, Y. L.; Zhang, Y. C.; Han, J. S.; Zhao, R. Y.; Zhao, J.; Wang, Z. H.; Zhao, Z. C.; Li, W. J.; Liu, F. S. Azobenzene based photo-responsive mechanical actuator fabricated by intermolecular H-bond interaction. Chinese J. Polym. Sci. 2021, 39, 417−424.
Photoinduced reversible solid-to-liquid transitions and directional photofluidization of azobenzene-containing polymers
Chinese J. Polym. Sci. 2020Liang, S. F.; Nie, C.; Yan, J.; Zhang, Q. J.; Wu, S. Photoinduced reversible solid-to-liquid transitions and directional photofluidization of azobenzene-containing polymers. Chinese J. Polym. Sci. 2020.
A straightforward protocol for the highly efficient preparation of main-chain azo polymers directly from bisnitroaromatic compounds by the photocatalytic process
Macromolecules 2015 48 1289 1295Wang, L. B.; Pan, X. Q.; Zhao, Y.; Chen, Y.; Zhang, W.; Tu, Y. F.; Zhang, Z. B.; Zhu, J.; Zhou, N. C.; Zhu, X. L. A straightforward protocol for the highly efficient preparation of main-chain azo polymers directly from bisnitroaromatic compounds by the photocatalytic process. Macromolecules 2015, 48, 1289−1295.
Design and synthesis of a novel azobenzene-containing polymer both in the main- and side-chain toward unique photocontrolled isomerization properties
Mater. Chem. Front. 2018 2 1112 1118Wang, K.; Yin, L.; Miu, T. F.; Liu, M.; Zhao, Y.; Chen, Y.; Zhou, N. C.; Zhang, W.; Zhu, X. L. Design and synthesis of a novel azobenzene-containing polymer both in the main- and side-chain toward unique photocontrolled isomerization properties. Mater. Chem. Front. 2018, 2, 1112−1118.
Unpolarized-light-driven amplified chiroptical modulation between chiral aggregation and achiral disaggregation of an azobenzene-alt-fluorene copolymer in limonene
Macromolecules 2011 44 5105 5111Zhang, W.; Yoshida, K.; Fujiki, M.; Zhu, X. L. Unpolarized-light-driven amplified chiroptical modulation between chiral aggregation and achiral disaggregation of an azobenzene-alt-fluorene copolymer in limonene. Macromolecules 2011, 44, 5105−5111.
Preparation of chiral and fluorescent nanoparticles of hyperbranched conjugated polymers by the solvent chirality transfer technology
Acta Polymerica Sinica (in Chinese) 2013 426 435Zhang, S. S.; Liu, J. F.; Zhang, J.; Wang, L. B.; Zhang, W.; Zhu, X. L. Preparation of chiral and fluorescent nanoparticles of hyperbranched conjugated polymers by the solvent chirality transfer technology. Acta Polymerica Sinica (in Chinese) 2013, 426−435.
Chiroptical generation and amplification of hyperbranched π-conjugated polymers in aggregation states driven by limonene chirality
Polym. Chem. 2014 5 784 791Liu, J. F.; Zhang, J.; Zhang, S. S.; Suzuki, N.; Fujiki, M.; Wang, L. B.; Li, L.; Zhang, W.; Zhou, N. C.; Zhu, X. L. Chiroptical generation and amplification of hyperbranched π-conjugated polymers in aggregation states driven by limonene chirality. Polym. Chem. 2014, 5, 784−791.
Limonene induced chiroptical generation and inversion during aggregation of achiral polyfluorene analogs: structure-dependence and mechanism
Polym. Chem. 2014 5 5920 5927Wang, L. B.; Suzuki, N.; Liu, J. F.; Matsuda, T.; Rahim, N. A. A.; Zhang, W.; Fujiki, M.; Zhang, Z. B.; Zhou, N. C.; Zhu, X. L. Limonene induced chiroptical generation and inversion during aggregation of achiral polyfluorene analogs: structure-dependence and mechanism. Polym. Chem. 2014, 5, 5920−5927.
Supramolecular chirality in achiral polyfluorene: chiral gelation, memory of chirality, and chiral sensing property
Macromolecules 2016 49 3214 3221Zhao, Y.; Rahirn, N. A. A.; Xia, Y. J.; Fujiki, M.; Song, B.; Zhang, Z. B.; Zhang, W.; Zhu, X. L. Supramolecular chirality in achiral polyfluorene: chiral gelation, memory of chirality, and chiral sensing property. Macromolecules 2016, 49, 3214−3221.
Helical screw sense bias in chiral polyfluorene stimulated by solvent
Chirality 2017 29 107 114Zhao, Y.; Yin, L.; Liu, J. J.; Chen, H. L.; Zhang, W. Helical screw sense bias in chiral polyfluorene stimulated by solvent. Chirality 2017, 29, 107−114.
Rapid limonene-induced mirror symmetry breaking in achiral polyfluorene containing pendant crown ether groups: enhanced by ion complexation
React. Funct. Polym. 2017 121 76 81Liu, J. J.; Zhao, Y.; Chen, H. L.; Zhang, Z. B.; Zhang, W.; Zhu, X. L. Rapid limonene-induced mirror symmetry breaking in achiral polyfluorene containing pendant crown ether groups: enhanced by ion complexation. React. Funct. Polym. 2017, 121, 76−81.
Chirality induction of achiral polydialkylfluorenes by chiral solvation: odd-even and side chain length dependence
Polym. Chem. 2018 9 2295 2301Zhao, Y.; Chen, H. L.; Yin, L.; Cheng, X. X.; Zhang, W.; Zhu, X. L. Chirality induction of achiral polydialkylfluorenes by chiral solvation: odd-even and side chain length dependence. Polym. Chem. 2018, 9, 2295−2301.
Different phase-dominated chiral assembly of polyfluorenes induced by chiral solvation: axial and supramolecular chirality
RSC Adv. 2019 9 38257 38264Li, S.; Miao, T. F.; Cheng, X. X.; Zhao, Y.; Zhang, W.; Zhu, X. L. Different phase-dominated chiral assembly of polyfluorenes induced by chiral solvation: axial and supramolecular chirality. RSC Adv. 2019, 9, 38257−38264.
Helically π-stacked thiophene-based copolymers with circularly polarized fluorescence: high dissymmetry factors enhanced by self-ordering in chiral nematic liquid crystal phase
Chem. Mater. 2012 24 1011 1024Watanabe, K.; Osaka, I.; Yorozuya, S.; Akagi, K. Helically π-stacked thiophene-based copolymers with circularly polarized fluorescence: high dissymmetry factors enhanced by self-ordering in chiral nematic liquid crystal phase. Chem. Mater. 2012, 24, 1011−1024.
Chiroptical properties of chiral substituted polyfluorenes
Macromolecules 2002 35 6792 6798Oda, M.; Nothofer, H. G.; Scherf, U.; Sunjic, V.; Richter, D.; Regenstein, W.; Neher, D. Chiroptical properties of chiral substituted polyfluorenes. Macromolecules 2002, 35, 6792−6798.
Circularly polarized electroluminescence from liquid-crystalline chiral polyfluorenes
Adv. Mater. 2000 12 362 365Oda, M.; Nothofer, H. G.; Lieser, G.; Scherf, U.; Meskers, S. C. J.; Neher, D. Circularly polarized electroluminescence from liquid-crystalline chiral polyfluorenes. Adv. Mater. 2000, 12, 362−365.
A programmable and biomimetic photo-actuator: a composite of a photo-liquefiable azobenzene derivative and commercial plastic film
J. Mater. Chem. C 2018 6 10815 10821Hu, J.; Li, X.; Ni, Y.; Ma, S. D.; Yu, H. F. A programmable and biomimetic photo-actuator: a composite of a photo-liquefiable azobenzene derivative and commercial plastic film. J. Mater. Chem. C 2018, 6, 10815−10821.
Flexible solar thermal fuel devices: composites of fabric and a photoliquefiable azobenzene derivative
Adv. Energy Mater. 2019 9 1901363Hu, J.; Huang, S.; Yu, M. M.; Yu, H. F. Flexible solar thermal fuel devices: composites of fabric and a photoliquefiable azobenzene derivative. Adv. Energy Mater. 2019, 9, 1901363.
Photomechanical motion of liquid-crystalline fibers bending away from a light source
Macromolecules 2017 50 8317 8324Cheng, Z. X.; Ma, S. D.; Zhang, Y. H.; Huang, S.; Chen, Y. X.; Yu, H. F. Photomechanical motion of liquid-crystalline fibers bending away from a light source. Macromolecules 2017, 50, 8317−8324.
A novel side-chain liquid crystal elastomer exhibiting anomalous reversible shape change
Angew. Chem. Int. Ed. 2020 59 15129 15134Yin, L.; Han, L.; Ge, F. J.; Tong, X.; Zhang, W.; Soldera, A.; Zhao, Y. A novel side-chain liquid crystal elastomer exhibiting anomalous reversible shape change. Angew. Chem. Int. Ed. 2020, 59, 15129−15134.
Sequentially adsorbed electrostatic multilayers of branched side-chain polyelectrolytes bearing donor-acceptor type Azo chromophores
Macromolecules 2004 37 135 146Wang, H. P.; He, Y. N.; Tuo, X. L.; Wang, X. G. Sequentially adsorbed electrostatic multilayers of branched side-chain polyelectrolytes bearing donor-acceptor type Azo chromophores. Macromolecules 2004, 37, 135−146.
Photoinduced mass-migration behavior of two amphiphilic side-chain azo diblock copolymers with different length flexible spacers
Macromolecules 2009 42 2651 2657Wang, D. R.; Ye, G.; Zhu, Y.; Wang, X. G. Photoinduced mass-migration behavior of two amphiphilic side-chain azo diblock copolymers with different length flexible spacers. Macromolecules 2009, 42, 2651−2657.
Photoinduced deformation of amphiphilic azo polymer colloidal spheres
J. Am. Chem. Soc. 2005 127 2402 2403Li, Y. B.; He, Y. N.; Tong, X. L.; Wang, X. G. Photoinduced deformation of amphiphilic azo polymer colloidal spheres. J. Am. Chem. Soc. 2005, 127, 2402−2403.
Fractal structures from amphiphilic random azo copolymer
Macromolecules 2011 44 8598 8606Li, N.; Li, Y. B.; Wang, X. G. Fractal structures from amphiphilic random azo copolymer. Macromolecules 2011, 44, 8598−8606.
Hybrid colloids composed of two amphiphilic azo polymers: fabrication, characterization, and photoresponsive properties
Macromolecules 2007 40 6669 6678Deng, Y. H.; Li, N.; He, Y. N.; Wang, X. G. Hybrid colloids composed of two amphiphilic azo polymers: fabrication, characterization, and photoresponsive properties. Macromolecules 2007, 40, 6669−6678.
Photomechanical effects of ferroelectric liquid-crystalline elastomers containing azobenzene chromophores
Angew. Chem. Int. Ed. 2007 46 881 883Yu, Y. L.; Maeda, T.; Mamiya, J.; Ikeda, T. Photomechanical effects of ferroelectric liquid-crystalline elastomers containing azobenzene chromophores. Angew. Chem. Int. Ed. 2007, 46, 881−883.
Photocontrol of fluid slugs in liquid crystal polymer microactuators
Nature 2016 537 179 184Lv, J. A.; Liu, Y. Y.; Wei, J.; Chen, E. Q.; Qin, L.; Yu, Y. L. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179−184.
Humidity- and photo-induced mechanical actuation of cross-linked liquid crystal polymers
Adv. Mater. 2017 29 1604792Liu, Y. Y.; Xu, B.; Sun, S. T.; Wei, J.; Wu, L. M.; Yu, Y. L. Humidity- and photo-induced mechanical actuation of cross-linked liquid crystal polymers. Adv. Mater. 2017, 29, 1604792.
Photomobile polymer materials: towards light-driven plastic motors
Angew. Chem. Int. Ed. 2008 47 4986 4988Yamada, M.; Kondo, M.; Mamiya, J. I.; Yu, Y. L.; Kinoshita, M.; Barrett, C. J.; Ikeda, T. Photomobile polymer materials: towards light-driven plastic motors. Angew. Chem. Int. Ed. 2008, 47, 4986−4988.
Photocontrollable induction of supramolecular chirality in achiral side chain Azo-containing polymers through preferential chiral solvation
Polym. Chem. 2015 6 4230 4239Jiang, S. Q.; Zhao, Y.; Wang, L.; Yin, L. B.; Zhang, Z. B.; Zhu, J.; Zhang, W.; Zhu, X. L. Photocontrollable induction of supramolecular chirality in achiral side chain Azo-containing polymers through preferential chiral solvation. Polym. Chem. 2015, 6, 4230−4239.
Supramolecular chirality induced by chiral solvation in achiral cyclic Azo-containing polymers: topological effects on chiral aggregation
Polym. Chem. 2018 9 769 776Yin, L.; Liu, M.; Zhao, Y.; Zhang, S. S.; Zhang, W.; Zhang, Z. B. A.; Zhu, X. L. Supramolecular chirality induced by chiral solvation in achiral cyclic Azo-containing polymers: topological effects on chiral aggregation. Polym. Chem. 2018, 9, 769−776.
Preferential chiral solvation induced supramolecular chirality in optically inactive star Azo polymers: photocontrollability, chiral amplification and topological effects
Polym. Chem. 2015 6 7045 7052Yin, L.; Zhao, Y.; Jiang, S. Q.; Wang, L. B.; Zhang, Z. B.; Zhu, J.; Zhang, W.; Zhu, X. L. Preferential chiral solvation induced supramolecular chirality in optically inactive star Azo polymers: photocontrollability, chiral amplification and topological effects. Polym. Chem. 2015, 6, 7045−7052.
Induction of supramolecular chirality by chiral solvation in achiral Azo polymers with different spacer lengths and push-pull electronic substituents: where will chiral induction appear?
Polym. Chem. 2017 8 1906 1913Yin, L.; Zhao, Y.; Liu, M.; Zhou, N. C.; Zhang, W.; Zhu, X. L. Induction of supramolecular chirality by chiral solvation in achiral Azo polymers with different spacer lengths and push-pull electronic substituents: where will chiral induction appear? Polym. Chem. 2017, 8, 1906−1913.
Chirality construction from preferred π-π stacks of achiral azobenzene units in polymer: chiral induction, transfer and memory
Polymers 2018 10 612Miao, T. F.; Yin, L.; Cheng, X. X.; Zhao, Y.; Hou, W. J.; Zhang, W.; Zhu, X. L. Chirality construction from preferred π-π stacks of achiral azobenzene units in polymer: chiral induction, transfer and memory. Polymers 2018, 10, 612.
Chiral induction by helical neighbour: spectroscopic visualization of macroscopic-interaction among self-sorted donor and acceptor π-stacks
Chem. Commun. 2011 47 8934 8936Molla, M. R.; Das, A.; Ghosh, S. Chiral induction by helical neighbour: spectroscopic visualization of macroscopic-interaction among self-sorted donor and acceptor π-stacks. Chem. Commun. 2011, 47, 8934−8936.
Enantioselective component selection in multicomponent supramolecular gels
J. Am. Chem. Soc. 2014 136 1116 1124Edwards, W.; Smith, D. K. Enantioselective component selection in multicomponent supramolecular gels. J. Am. Chem. Soc. 2014, 136, 1116−1124.
Self-assembly of tris(phenylisoxazolyl) benzene and its asymmetric induction of supramolecular chirality
Chem. Commun. 2008 468 470Haino, T.; Tanaka, M.; Fukazawa, Y. Self-assembly of tris(phenylisoxazolyl) benzene and its asymmetric induction of supramolecular chirality. Chem. Commun. 2008, 468−470.
Gelating-induced supramolecular chirality of achiral porphyrins: chiroptical switch between achiral molecules and chiral assemblies
Soft Matter 2007 3 1312 1317Li, Y. G.; Wang, T. Y.; Liu, M. H. Gelating-induced supramolecular chirality of achiral porphyrins: chiroptical switch between achiral molecules and chiral assemblies. Soft Matter 2007, 3, 1312−1317.
A strategy for tuning achiral main-chain polymers into helical assemblies and chiral memory systems
Soft Matter 2016 12 1170 1175Yang, D.; Zhao, Y.; Lv, K.; Wang, X. F.; Zhang, W.; Zhang, L.; Liu, M. H. A strategy for tuning achiral main-chain polymers into helical assemblies and chiral memory systems. Soft Matter 2016, 12, 1170−1175.
Fabrication of chiroptically switchable films via co-gelation of a small chiral gelator with an achiral azobenzene-containing polymer
Soft Matter 2017 13 6129 6136Yang, D.; Zhang, L.; Yin, L.; Zhao, Y.; Zhang, W.; Liu, M. H. Fabrication of chiroptically switchable films via co-gelation of a small chiral gelator with an achiral azobenzene-containing polymer. Soft Matter 2017, 13, 6129−6136.
Self-induced light polarization rotation in azobenzene-containing polymers
Appl. Phys. Lett. 2000 77 657 659Nikolova, L.; Nedelchev, L.; Todorov, T.; Petrova, T.; Tomova, N.; Dragostinova, V.; Ramanujam, P. S.; Hvilsted, S. Self-induced light polarization rotation in azobenzene-containing polymers. Appl. Phys. Lett. 2000, 77, 657−659.
Photoinduced chirality in azobenzene-containing polymer systems
Phys. Chem. Chem. Phys. 2007 9 3671 3681Choi, S. W.; Kawauchi, S.; Ha, N. Y.; Takezoe, H. Photoinduced chirality in azobenzene-containing polymer systems. Phys. Chem. Chem. Phys. 2007, 9, 3671−3681.
Control of chirality of an azobenzene liquid crystalline polymer with circularly polarized light
J. Am. Chem. Soc. 2000 122 12646 12650Iftime, G.; Labarthet, F. L.; Natansohn, A.; Rochon, P. Control of chirality of an azobenzene liquid crystalline polymer with circularly polarized light. J. Am. Chem. Soc. 2000, 122, 12646−12650.
Photoinduced supramolecular chirality in amorphous azobenzene polymer films
J. Am. Chem. Soc. 2002 124 3504 3505Kim, M. J.; Shin, B. G.; Kim, J. J.; Kim, D. Y. Photoinduced supramolecular chirality in amorphous azobenzene polymer films. J. Am. Chem. Soc. 2002, 124, 3504−3505.
Bent-core liquid crystals: their mysterious and attractive world
Jpn. J. Appl. Phys. 2006 45 597 625Takezoe, H.; Takanishi, Y. Bent-core liquid crystals: their mysterious and attractive world. Jpn. J. Appl. Phys. 2006, 45, 597−625.
Control and modulation of chirality for azobenzene-substituted polydiacetylene LB films with circularly polarized light
Chem. Commun. 2009 5627 5629Zou, G.; Jiang, H.; Kohn, H.; Manaka, T.; Iwamoto, M. Control and modulation of chirality for azobenzene-substituted polydiacetylene LB films with circularly polarized light. Chem. Commun. 2009, 5627−5629.
Improvement of photoinduced birefringence properties of optically active methacrylic polymers through copolymerization of monomers bearing azoaromatic moieties
Macromolecules 2006 39 489 497Angiolini, L.; Benelli, T.; Giorgini, L.; Salatelli, E.; Bozio, R.; Dauru, A.; Pedron, D. Improvement of photoinduced birefringence properties of optically active methacrylic polymers through copolymerization of monomers bearing azoaromatic moieties. Macromolecules 2006, 39, 489−497.
Mirror symmetry breaking and restoration within μm-sized polymer particles in optofluidic media by pumping circularly polarised light
RSC Adv. 2013 3 5213 5219Fujiki, M.; Yoshida, K.; Suzuki, N.; Zhang, J.; Zhang, W.; Zhu, X. L. Mirror symmetry breaking and restoration within μm-sized polymer particles in optofluidic media by pumping circularly polarised light. RSC Adv. 2013, 3, 5213−5219.
Photon magic: chiroptical polarisation, depolarisation, inversion, retention and switching of non-photochromic light-emitting polymers in optofluidic medium
Polym. Chem. 2015 6 1627 1638Fujiki, M.; Donguri, Y.; Zhao, Y.; Nakao, A.; Suzuki, N.; Yoshida, K.; Zhang, W. Photon magic: chiroptical polarisation, depolarisation, inversion, retention and switching of non-photochromic light-emitting polymers in optofluidic medium. Polym. Chem. 2015, 6, 1627−1638.
Tricks of light on helices: transformation of helical polymers by photoirradiation
Chem. Rec. 2014 14 369 385Nakano, T. Tricks of light on helices: transformation of helical polymers by photoirradiation. Chem. Rec. 2014, 14, 369−385.
Helix induction to polyfluorenes using circularly polarized light: chirality amplification, phase-selective induction, and anisotropic emission
Macromolecules 2018 51 6865 6877Wang, Y.; Harada, T.; Phuong, L. Q.; Kanemitsu, Y.; Nakano, T. Helix induction to polyfluorenes using circularly polarized light: chirality amplification, phase-selective induction, and anisotropic emission. Macromolecules 2018, 51, 6865−6877.
Circularly polarized light with sense and wavelengths to regulate azobenzene supramolecular chirality in optofluidic medium
J. Am. Chem. Soc. 2017 139 13218 13226Wang, L. B.; Yin, L.; Zhang, W.; Zhu, X. L.; Fujiki, M. Circularly polarized light with sense and wavelengths to regulate azobenzene supramolecular chirality in optofluidic medium. J. Am. Chem. Soc. 2017, 139, 13218−13226.
Color indicators of molecular chirality based on doped liquid crystals
Angew. Chem. Int. Ed. 2001 40 3198 3200van, Delden R. A.; Feringa, B. L. Color indicators of molecular chirality based on doped liquid crystals. Angew. Chem. Int. Ed. 2001, 40, 3198−3200.
Chirality transfer in ferroelectric liquid crystals
Acc. Chem. Res. 2001 34 845 853Lemieux, R. P. Chirality transfer in ferroelectric liquid crystals. Acc. Chem. Res. 2001, 34, 845−853.
Light-driven nanoscale chiral molecular switch: reversible dynamic full range color phototuning
Chem. Commun. 2010 46 3463 3465Ma, J.; Li, Y. N.; White, T.; Urbas, A.; Li, Q. Light-driven nanoscale chiral molecular switch: reversible dynamic full range color phototuning. Chem. Commun. 2010, 46, 3463−3465.
Formation of helical phases in achiral block copolymers by simple addition of small chiral additives
Macromolecules 2014 47 6547 6553Yao, L.; Lu, X.; Chen, S.; Watkins, J. J. Formation of helical phases in achiral block copolymers by simple addition of small chiral additives. Macromolecules 2014, 47, 6547−6553.
Chiroptical nanofibers generated from achiral metallophthalocyanines induced by diamine homochirality
Chem. Eur. J. 2011 17 10628 10635Zhang, W.; Fujiki, M.; Zhu, X. L. Chiroptical nanofibers generated from achiral metallophthalocyanines induced by diamine homochirality. Chem. Eur. J. 2011, 17, 10628−10635.
The construction of photoresponsive polymer particles with supramolecular helicity from achiral monomers by helix-sense-selective polymerization
Polym. Chem. 2020 11 2089 2097Cheng, X. X.; Miao, T. F.; Ma, H. T.; Yin, L.; Zhang, W.; Zhang, Z. B.; Zhu, X. L. The construction of photoresponsive polymer particles with supramolecular helicity from achiral monomers by helix-sense-selective polymerization. Polym. Chem. 2020, 11, 2089−2097.
Macromolecular multi-chromophoric scaffolding
Chem. Soc. Rev. 2010 39 1576 1599Schwartz, E.; Le, Gac S.; Cornelissen, J. J. L. M.; Nolte, R. J. M.; Rowan, A. E. Macromolecular multi-chromophoric scaffolding. Chem. Soc. Rev. 2010, 39, 1576−1599.
Oligosilane-nanofibers can be prepared through fabrication of permethyldecasilane within a helical superstructure of schizophyllan
Org. Lett. 2005 7 5605 5608Haraguchi, S.; Hasegawa, T.; Numata, M.; Fujiki, M.; Uezu, K.; Sakurai, K.; Shinkai, S. Oligosilane-nanofibers can be prepared through fabrication of permethyldecasilane within a helical superstructure of schizophyllan. Org. Lett. 2005, 7, 5605−5608.
Beta-1,3-glucan (Schizophyllan) can act as a one-dimensional host for creation of novel poly(aniline) nanofiber structures
Org. Lett. 2004 6 4447 4450Numata, M.; Hasegawa, T.; Fujisawa, T.; Sakurai, K.; Shinkai, S. Beta-1,3-glucan (Schizophyllan) can act as a one-dimensional host for creation of novel poly(aniline) nanofiber structures. Org. Lett. 2004, 6, 4447−4450.
Self-assembly of supramolecular chiral insulated molecular wire
J. Am. Chem. Soc. 2005 127 4548 4549Li, C.; Numata, M.; Bae, A. H.; Sakurai, K.; Shinkai, S. Self-assembly of supramolecular chiral insulated molecular wire. J. Am. Chem. Soc. 2005, 127, 4548−4549.
Oligo- and polyfluorenes meet cellulose alkyl esters: Retention, inversion, and racemization of circularly polarized luminescence (CPL) and circular dichroism (CD) via intermolecular C-H/O=C interactions
Macromolecules 2017 50 1778 1789Guo, S. B.; Suzuki, N.; Fujiki, M. Oligo- and polyfluorenes meet cellulose alkyl esters: Retention, inversion, and racemization of circularly polarized luminescence (CPL) and circular dichroism (CD) via intermolecular C-H/O=C interactions. Macromolecules 2017, 50, 1778−1789.
DNA-guided plasmonic helix with switchable chirality
J. Am. Chem. Soc. 2018 140 11763 11770Lan, X.; Liu, T. J.; Wang, Z. M.; Govorov, A. O.; Yan, H.; Liu, Y. DNA-guided plasmonic helix with switchable chirality. J. Am. Chem. Soc. 2018, 140, 11763−11770.
The origin of bisignate circularly polarized luminescence (CPL) spectra from chiral polymer aggregates and molecular camphor: anti-Kasha's rule revealed by CPL excitation (CPLE) spectra
Polym. Chem. 2017 8 4673 4679Duong, S. T.; Fujiki, M. The origin of bisignate circularly polarized luminescence (CPL) spectra from chiral polymer aggregates and molecular camphor: anti-Kasha's rule revealed by CPL excitation (CPLE) spectra. Polym. Chem. 2017, 8, 4673−4679.
Time-evolved, far-red, circularly polarised luminescent polymer aggregates endowed with sacrificial helical Si-Si bond polymers
Mater. Chem. Front. 2017 1 1773 1785Fujiki, M.; Yoshimoto, S. Time-evolved, far-red, circularly polarised luminescent polymer aggregates endowed with sacrificial helical Si-Si bond polymers. Mater. Chem. Front. 2017, 1, 1773−1785.
Aggregation-induced scaffolding: Photoscissable helical polysilane generates circularly polarized luminescent polyfluorene
Polym. Chem. 2016 7 4618 4629Rahim, N. A. A.; Fujiki, M. Aggregation-induced scaffolding: Photoscissable helical polysilane generates circularly polarized luminescent polyfluorene. Polym. Chem. 2016, 7, 4618−4629.
Aggregation-induced chiroptical generation and photoinduced switching of achiral azobenzene-alt-fluorene copolymer endowed with left- and right-handed helical polysilanes
RSC Adv. 2019 9 4849 4856Chen, H. L.; Yin, L.; Liu, M.; Wang, L. B.; Fujiki, M.; Zhang, W.; Zhu, X. L. Aggregation-induced chiroptical generation and photoinduced switching of achiral azobenzene-alt-fluorene copolymer endowed with left- and right-handed helical polysilanes. RSC Adv. 2019, 9, 4849−4856.
Self-dopant formation in poly(9,9-di-n-octylfluorene) via a dipping method for efficient and stable pure-blue electrolumineseence
Adv. Mater. 2007 19 2574 2579Lu, H. H.; Liu, C. Y.; Chang, C. H.; Chen, S. A. Self-dopant formation in poly(9,9-di-n-octylfluorene) via a dipping method for efficient and stable pure-blue electrolumineseence. Adv. Mater. 2007, 19, 2574−2579.
Supramolecular chiral structures: smart polymer organization guided by 2D polarization light patterns
Adv. Funct. Mater. 2012 22 2964 2970Ruiz, U.; Pagliusi, P.; Provenzano, C.; Shibaev, V. P.; Cipparrone, G. Supramolecular chiral structures: smart polymer organization guided by 2D polarization light patterns. Adv. Funct. Mater. 2012, 22, 2964−2970.
Supramolecular chiroptical switches
Chem. Soc. Rev. 2020Zhang, L.; Wang, H. X.; Li, S.; Liu, M. H. Supramolecular chiroptical switches. Chem. Soc. Rev. 2020.
Circularly polarized luminescence in chiral molecules and supramolecular assemblies
J. Phys. Chem. Lett. 2015 6 3445 3452Kumar, J.; Nakashima, T.; Kawai, T. Circularly polarized luminescence in chiral molecules and supramolecular assemblies. J. Phys. Chem. Lett. 2015, 6, 3445−3452.
Circularly polarized luminescence in nanoassemblies: generation, amplification, and application
Adv. Mater. 2020 32 1900110Sang, Y. T.; Han, J. L.; Zhao, T. H.; Duan, P. F.; Liu, M. H. Circularly polarized luminescence in nanoassemblies: generation, amplification, and application. Adv. Mater. 2020, 32, 1900110.
In situ controlled construction of a hierarchical supramolecular chiral liquid-crystalline polymer assembly
Angew. Chem. Int. Ed. 2020 59 9669 9677Cheng, X. X.; Miao, T. F.; Yin, L.; Ji, Y. J.; Li, Y. Y.; Zhang, Z. B.; Zhang, W.; Zhu, X. L. In situ controlled construction of a hierarchical supramolecular chiral liquid-crystalline polymer assembly. Angew. Chem. Int. Ed. 2020, 59, 9669−9677.
Polymerization-induced self-assembly of conjugated block copoly(phenylacetylene)s
Macromolecules 2020 53 1638 1644Chen, J. X.; Cai, S. L.; Wang, R.; Wang, S.; Zhang, J.; Wan, X. H. Polymerization-induced self-assembly of conjugated block copoly(phenylacetylene)s. Macromolecules 2020, 53, 1638−1644.
Nickel-catalyzed coordination polymerization-induced self-assembly of helical poly(aryl isocyanide)s
ACS Macro Lett. 2020 9 226 232Jimaja, S.; Varlas, S.; Xie, Y. J.; Foster, J. C.; Taton, D.; Dove, A. P.; O'Reilly, R. K. Nickel-catalyzed coordination polymerization-induced self-assembly of helical poly(aryl isocyanide)s. ACS Macro Lett. 2020, 9, 226−232.