INTRODUCTION
Bacteria-associated infections are posing an unprecedented threat to human health worldwide.[1] It is estimated that, if no effective measures are taken, bacterial infection will surpass cancer and become the number one cause of human death by 2050.[2] The frightening scenario of multidrug resistance (MDR) was partly promoted by the abuse and misuse of antibiotics and partly promoted by the ineffectiveness of the antibiotic regimens against bacterial biofilms.[3] Biofilms are well-structured bacterial communities in which bacterial cells aggregate in the self-produced extracellular polymeric substances (EPS) consisting of polysaccharides, proteins, glycoproteins, and nucleic acids.[4] The porous nature of an EPS matrix allows the penetration of nutrients and transport of wastes for the metabolic activities of the inhabitants.[5] In the meantime, biofilms protect the inhabitants against the host immune systems and environmental challenges,[4] as well as antibiotic pressure.[3] Hydrophobic antibiotics barely penetrate bacterial biofilms due to their poor solubility. Besides, most of the commonly used antibiotics carry a positive net charge, and thereby, the negatively charged EPS matrix can absorb those cationic antibiotics through electrostatic attractions and hinder their deep penetration in biofilms. For those antibiotics that penetrate in biofilms, they may face the possibility of being pumped out by the over-expressed drug efflux pumps in biofilms[6] or being hydrolysed by the local bacterial enzymes such as β-lactamases.[7] Also, antibiotics are less effective for the bacteria in their biofilm-phenotype that are featured by their reduced metabolic and growth activities compared with their planktonic counterparts. Taken together, all of the above reasons lead to the low efficiency of traditional antibiotics in eradicating biofilms. Usually, up to 1000 times more dosage of antibiotics are required to treat bacteria in biofilms than their planktonic counterparts, ultimately expediting the development of antimicrobial resistance.[8]
The development of new strategies for biofilm-associated infection control is eagerly needed, either to develop new antibiotics or to improve the bactericidal efficacy of the current antibiotic regimens. Unfortunately, the lifetime of new antibiotics is becoming increasingly shorter since the emergence of drug resistance in bacteria. In this regard, the motivation to develop and market new antibiotics with huge research costs is decreasing.[9] To solve the dilemma, over the past decades, numerous nanotechnology-based systems have been designed as antimicrobials and delivery systems to facilitate the penetration and drug release in biofilms, such as metal-based nanocomposites (e.g., metal oxide-, Ag-, and Au-based nanoparticles), carbon-based nanomaterials (e.g., graphene materials, carbon quantum dots), and polymer-based nanoparticles (natural and synthetic polymeric nanoparticles).[10,11] Among them, synthetic polymeric nanoparticles are flourishing, since the development of polymerization methodologies allows the synthesis of polymers with precise structures and molecular sizes.[12-15] Also, there are multitudinous functional groups that could be modified on the polymer chains via post-polymerization modification, endowing the polymer with different physicochemical properties applicable in the biofilm microenvironment, such as biocompatibility, stimuli-responsiveness and degradability.[16]
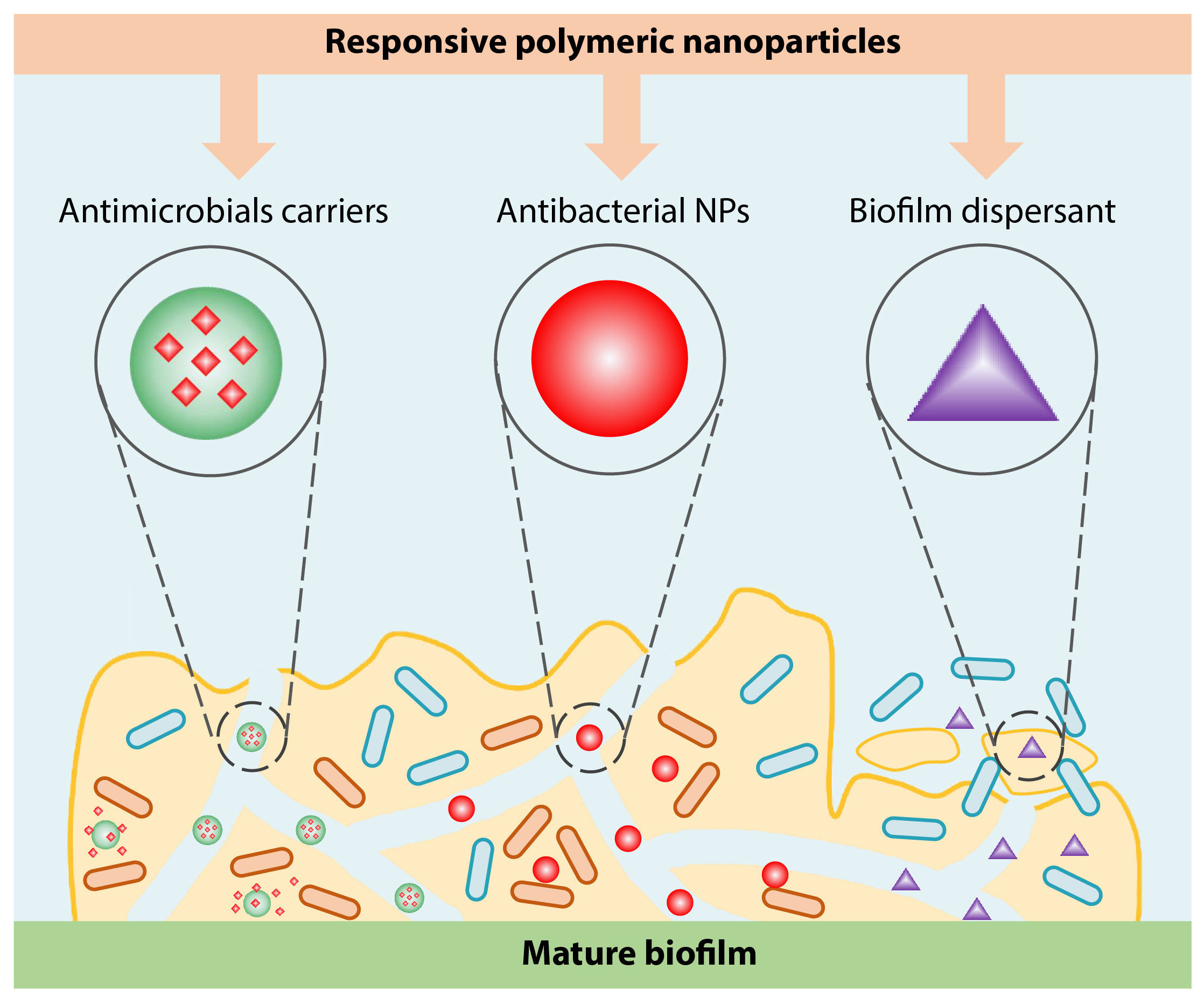
Polymer nanosystems can be applied in combating biofilm-associated infections in two major aspects, as summarized in Scheme 1. First, polymers can be used to modify the surfaces of substrates to avoid bacterial adhesion and subsequently prevent biofilm formation, yielding the antifouling surfaces or antibacterial surfaces. Besides, mature biofilms are eradicated by polymer nanosystems from different perspectives. With rational design, bactericidal polymers can be armed with intrinsic antibacterial capacities, for example, quaternary ammonium-containing polymers,[17-21] polycations,[22-26] polysaccharides[27] and antimicrobial peptides (AMPs).[28-30] Moreover, polymeric nanocarriers can deliver antimicrobials deep into biofilms, enhance the penetration and accumulation of antimicrobials, and release the loaded antimicrobials inside biofilms, improving the efficacy of conventional antimicrobials yet minimizing their side effects on normal tissues.[31] Besides, polymeric nanoparticles may serve as a biofilm dispersant, rendering an efficient way to eradicate biofilms. In this regard, once the EPS matrix is dispersed, antimicrobials will exhibit better bactericidal efficacy against the remaining biofilms. In this perspective, we briefly focus on the recent development of polymer-based materials in eradicating biofilm and their potential clinical applications. Also, we discuss the challenges in their clinical translation.
MICROENVIRONMENT AT INFECTION SITES
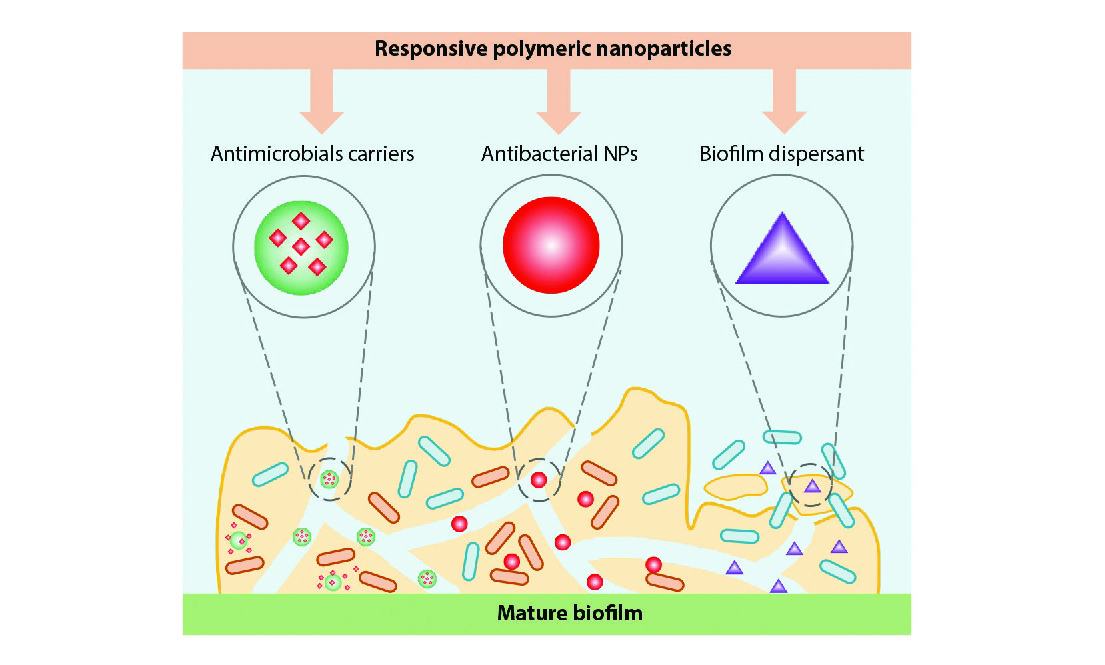
The metabolism activities of microorganisms embedded in infectious biofilms lead to the establishment of unique chemical and physical properties at infection sites, such as the leaky blood vessels, acidic environment, oxygen gradients, diverse enzymes, high levels of H2O2, and toxins. These characteristics not only discriminate the infection sites from the normal tissues but also give implications for designing smart antimicrobial systems (Fig. 1). In this section, we focus on the crucial microenvironment at infection sites and their functions in antimicrobial systems.
Enhanced Permeability and Retention (EPR) Effect
The EPR effect is a major effect known as that nanoparticles are allowed to accumulate in tumor tissue other than normal tissue.[32] This is because, in normal tissues, the endothelial space of micro-vessels is dense and intact, and nanoparticles are less likely to bypass the vascular wall. However, in those diseased tissues, the vascular wall space is much wider, leading to the permeability and retention of nanomedicines.[33-36] Of note, the EPR effect was observed not only in tumor tissues, but also in bacterial infection, inflammation, and injury sites.[37,38] During bacterial infection, proteases play an important role in forming leaky vascular structures.[39-42] The bradykinin-generating cascade of endogenous proteases is activated by exogenous proteases. Then, the endogenous proteases can activate thrombin, form fibrin and generate kinin, leading to the increase in vascular permeability.[38] Therefore, this vascular change allows nanoparticles to accumulate at the infection sites via the EPR effect. Particularly, the EPR effect lay the basis for the selective distribution of nanoparticles in bacterial infection, which can increase efficacy and reduce systemic side effects of conventional antimicrobials.
Acidic Environment
An acidic environment exists in a biofilm, in which the pH value in a biofilm may reach 4.5 or even lower.[43] For example, the metabolic activity of acidogenic/acid-tolerant oral bacteria can result in an acidic microenvironment.[44] The anaerobic fermentation of certain microbes, such as Staphylococcus aureus (S. aureus) or Streptococcus mutans (S. mutans), can produce and accumulate organic acids in biofilms, leading to an acidic environment.[45] Besides, bacterial infections can induce phagocytosis, during which, the inflammatory cells release lactic acid, lowering the pH at infection sites.[46] The low pH at infection sites is different from the physiological pH of 7.4, which can provide implications for the determination and treatment of bacterial infection.
In principle, several chemical groups can transform between protonation and de-protonation when the pH varies around their pKa values, such as amines, carboxylates and sulfonic groups.[47] In the meantime, the solubility of these chemical groups will change drastically, ultimately leading to the phase change of the bulk materials. Therefore, this property can be used to design pH-responsive nanomaterials for various purposes.[45]
Oxygen Gradients
The EPS matrix in biofilm leads to the creation of physio-chemical heterogeneities, for example, nutrient and oxygen gradients.[48] The oxygen gradient in a biofilm is attributed to the fact that bacteria in the superficial layers of the biofilm consume oxygen at a rate faster than the diffusion of oxygen, leading to the formation of hypoxia in the bottom layers of a biofilm.[49] This oxygen gradient and local hypoxia are of great importance to the metabolism, virulence, and drug resistance of bacteria.[5] Besides, the local hypoxia of a biofilm implicates the possibility of delivering and releasing antibiotics in an “on-demand” manner. The commonly used hypoxia-responsive moieties include azo crosslinkers, nitroimidazoles, and nitrobenzyl alcohols (Fig. 1).[50] In most cases, they undergo a hydrophobic-to-hydrophilic transition once being reduced under the hypoxia conditions. Those moieties have been extensively applied to design/construct controlled release systems in cancer therapy and immunotherapy.[51] Whereas, the application of hypoxia-responsive systems in combating bacteria-associated infections is still in its infancy. Besides, the oxygen-loaded nanocarriers can relieve the biofilm hypoxia to a certain extent.[52]
Enzymes
Like hypoxia, bacterial enzymes also play crucial roles in the metabolism, biological process, virulence, and drug resistance of bacteria.[53] There are several over-expressed enzymes at the infection sites, such as β-galactosidases,[54] alkaline phosphatases,[29,55] nitroreductases,[56] proteinases,[57] lipases,[58] phospholipases,[59] toxins,[60,61] and hyaluronidases.[62] These enzymes can degrade/hydrolyze specific chemical structures, potentiating their applications in drug delivery.[47]
Reactive Oxygen Species (ROS)
At the infection sites, the infiltrating immune cells produce an increased amount of ROS, such as hydrogen peroxide (H2O2), superoxide (O2–), hypochlorite (OCl–), etc. to eradicate the invaded bacteria.[63-65] Therefore, infection sites are often enriched with ROS that can be used to determine and eradicate bacterial infections. In this regard, numerous ROS-responsive systems have been investigated for diverse applications,[65-67] while their application in eradicating bacteria- or biofilm-associated infections remains largely undeveloped.
PREVENTION OF BIOFILM FORMATION
Bacteria need to adhere to the surface of tissues or implants and to initiate the growth of biofilms thereafter.[68,69] Therefore, the easiest and most convenient way to avoid biofilm-associated infections is to prevent biofilm formation. In general, there are two major approaches to prevent bacterial adhesion and subsequent formation of biofilms on a surface, namely to “attack” or to “defend”.[70] The “attack” strategy is to kill the bacteria using antibiotics or antimicrobial cationic polymers,[71-73] since the cell membranes of bacteria are negatively charged because of the ample anionic lipids, like phosphatidylglycerols and cardiolipins.[74] However, the dead bacteria will remain on these surfaces, which can trigger the immune response and induce side effects such as inflammation and sepsis. In the meanwhile, the “defend” strategy is to form a non-fouling surface using polymers such as poly(ethylene glycol) (PEG) and zwitterionic polymers.[75,76] In particular, these polymers can form a hydration periphery that can largely avoid the interactions of bacteria with the surface of a substratum. Of note, some recent developments realize the design of multifunctional surfaces. For example, zwitterionic surfaces formed by an equal amount of quaternary ammonium and carboxyl groups were able to achieve the “attract-and-release” of bacteria.[76,77] The zwitterionic surface is positively charged at pH below 4.5 due to the partial protonation of carboxyl groups, enabling the attraction of bacteria. While under neutral and basic conditions, the zwitterionic surface becomes neutral owing to the deprotonation of acids. Thus, the attenuated interaction between surface and bacteria leads to the release of the captured bacteria.[77] However, the pH variation only allows the switch of surfaces from bacteria-adhesive to bacteria-resistant, and the surfaces do not induce the lysis of bacterial cells. Subsequently, Jiang et al. developed a surface modified with ester-containing quaternary ammoniums to achieve the “kill-and-release” property.[78] The antibacterial surface possessed a strong positive charge that kills the attached bacteria by damaging the bacteria cell membrane. In the meantime, the released esterases from the killed bacteria could hydrolyze the ester groups, leading to the formation of a zwitterionic and non-fouling surface that liberates the dead bacteria.[78] However, the hydrolysis of ester groups is not reversible, and the surface cannot turn back its “kill” state to maintain its non-fouling nature. To solve this problem, in their subsequent study, a surface capable of switching between attacking and defending functions was developed. The surfaces can transform the cationic N,N-dimethyl-2-morpholinone into the zwitterionic carboxy betaine under the trigger of bacterial enzyme-triggered hydrolysis.[70]
Apart from the chemical modification of surfaces to prevent bacterial adhesion, the roughness and topography of substrate surfaces also influence bacterial adhesion, as reviewed elsewhere.[79,80] Polymer-based nanoparticles, such as nanogels, can form the antifouling nanogel coating on the surface of a substratum to reduce bacterial attachment via its unique topography and stiffness, as well as hydration property.[81] For example, poly(N-isopropylmetacrylamide) (pNIPMAM) nanogel coatings were used to prevent the adhesion of bacteria and subsequently prevent the formation of biofilms.[82] However, in most cases, only repelling the bacterial attachment is not enough to prevent the formation of biofilms.[83] To address this issue, antimicrobials are applied as additives to enhance the bactericidal efficacy and the ability to prevent biofilm formation. Antimicrobials can be loaded, chemically conjugated, or coordinated into the polymeric hydration layer or nanogels that coat on a surface. For example, Xu et al. designed porous hydroxyapatite (HA) implants functionalized via ethylenediamine-modified poly(glycidyl methacrylate) brushes, which further conjugated gentamicin sulfate (GS), yielding the antibacterial HA implants (HA-GS).[84] The local acidic microenvironment can trigger the release of GS via the hydrolysis of the acid-sensitive linkers between GS and polymer brushes. HA-GS exhibited excellent anti-infection activity in vivo in an infected bone defect rabbit model. Taken together, all the above-mentioned strategies provide powerful arsenals for preventing bacterial adhesion and subsequent biofilm formation.
BIOFILM ERADICATION
Besides preventing biofilm formation, eradicating the established biofilm is also a huge task. There are two main strategies to eradicate biofilm. On the one hand, polymer nanosystems can be used as carriers for traditional antibiotics or serve as nano-antibiotics to kill bacteria in biofilms. On the other hand, polymer nanoparticles can be used as biofilm dispersants to destroy the architectures of biofilms, making the remaining bacterial more susceptible to other antibacterial therapies, such as antibiotics. In this section, recent developments of polymer-based materials that have been applied in eradicating mature bacterial biofilms will be discussed.
Antibiotics Delivery Systems
Free antibiotics are easily cleared through blood circulation via renal filtration or inactivated by the various enzymes in tissues such as livers. Furthermore, as we mentioned above, the penetration of free antibiotics in biofilms is remarkably poor, greatly attenuating the anti-biofilm efficacy of antibiotic regimens and resulting in serious side effects. To solve this problem, conventional antibiotics can be loaded in polymeric nanoparticles. The drug-encapsulated polymeric systems possess the following advantages. First, after being encapsulated, conventional antibiotics are temporarily protected by the polymeric peripheries, thereby enhancing the stability and biocompatibility of antibiotics during blood circulation. Besides, certain polymeric carriers can respond to the bacterial infection microenvironments and target the infection sites. Moreover, with rational design, polymeric carriers can enhance the penetration of antibiotics in biofilms, and release their cargoes to eradicate the biofilms in an on-demand manner. Both hydrophilic and hydrophobic antibiotics can be loaded by choosing different polymer systems.
Loading hydrophobic antibiotics
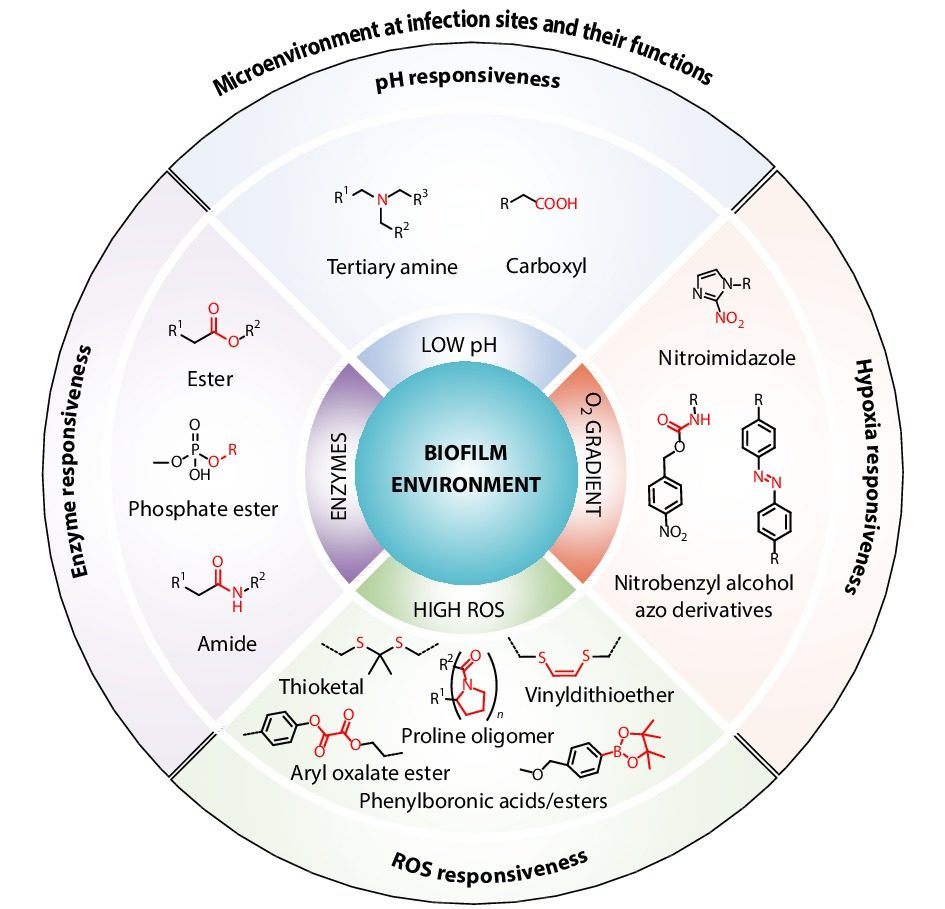
The most prevalent polymeric nanocarriers for hydrophobic antibiotics are polymeric micelles. In recent years, our group have made a lot of efforts to research polymer assembly behavior and its applications.[85-91] In particular, polymeric micelles are core-shell-structured nano-assemblies that self-assembled from amphiphilic copolymers. Therefore, the hydrophobic cores of polymeric micelles are the ideal containers for hydrophobic antibiotics, such as triclosan,[92] curcumin,[93] isoniazid,[94] and bedaquiline.[95] There are several approaches for the preparation of the hydrophobic antimicrobial-loaded polymeric micelles, which have been reviewed elsewhere.[16,47] This is not the subject of our article. We merely focus on the utility of polymeric micelles in overcoming the physical barrier of biofilm during antibiotic treatments. For example, triclosan could be loaded in mixed-shell-polymeric-micelles (MSPMs), which are self-assembled from two diblock copolymers, namely poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-b-PCL) and poly(ε-caprolactone)-block-poly(β-amino ester) (PCL-b-PAE) (Fig. 2).[92] The shells of MSPMs are formed by the hydrophilic PEG and the pH-responsive PAE,[96] while PCL forms the lipase-sensitive micelle core that can encapsulate hydrophobic triclosan. The mixed shell allows the stability and long circulation of MSPMs, since the PAE block is negatively charged and hydrophobic under normal physiological pH 7.4, and the whole micelles were stabilized by the PEG shell.[92] Once being exposed to the acidic microenvironment of infection sites, the PAE block would be protonated and become hydrophilic and positively charged, enhancing their interactions with the negatively charged bacteria in biofilms through electrostatic attractions. Upon the deep penetration of the drug-loaded MSPMs in biofilms, the micellar cores would be hydrolyzed by the bacterial lipases, releasing the loaded triclosan to kill the bacteria embedded in biofilms. In contrast, non-loaded triclosan and the triclosan encapsulated in single-shell-polymeric-micelles (SSPMs) consisting of only PEG-b-PCL exhibited poor biofilm eradication efficacy. This study renders an efficient pathway to overcome the biofilm barrier.
Subsequently, we used MSPMs to encapsulate protoporphyrin IX (PpIX), a photosensitizer that produces singlet oxygen using tissue oxygen under light irradiation, for biofilm eradication. Our MSPMs greatly enhanced the penetration of the hydrophobic PpIX in biofilms and the ability to produce singlet oxygen thereafter.[97] Since singlet oxygen induces bacterial lysis using its oxidative stress on bacterial lipids and biomacromolecules, bacteria are less likely resistant to this mechanism. Therefore, these photosensitizer-based bactericidal systems are ready to be applied in combating biofilms constructed from drug-resistant bacteria.
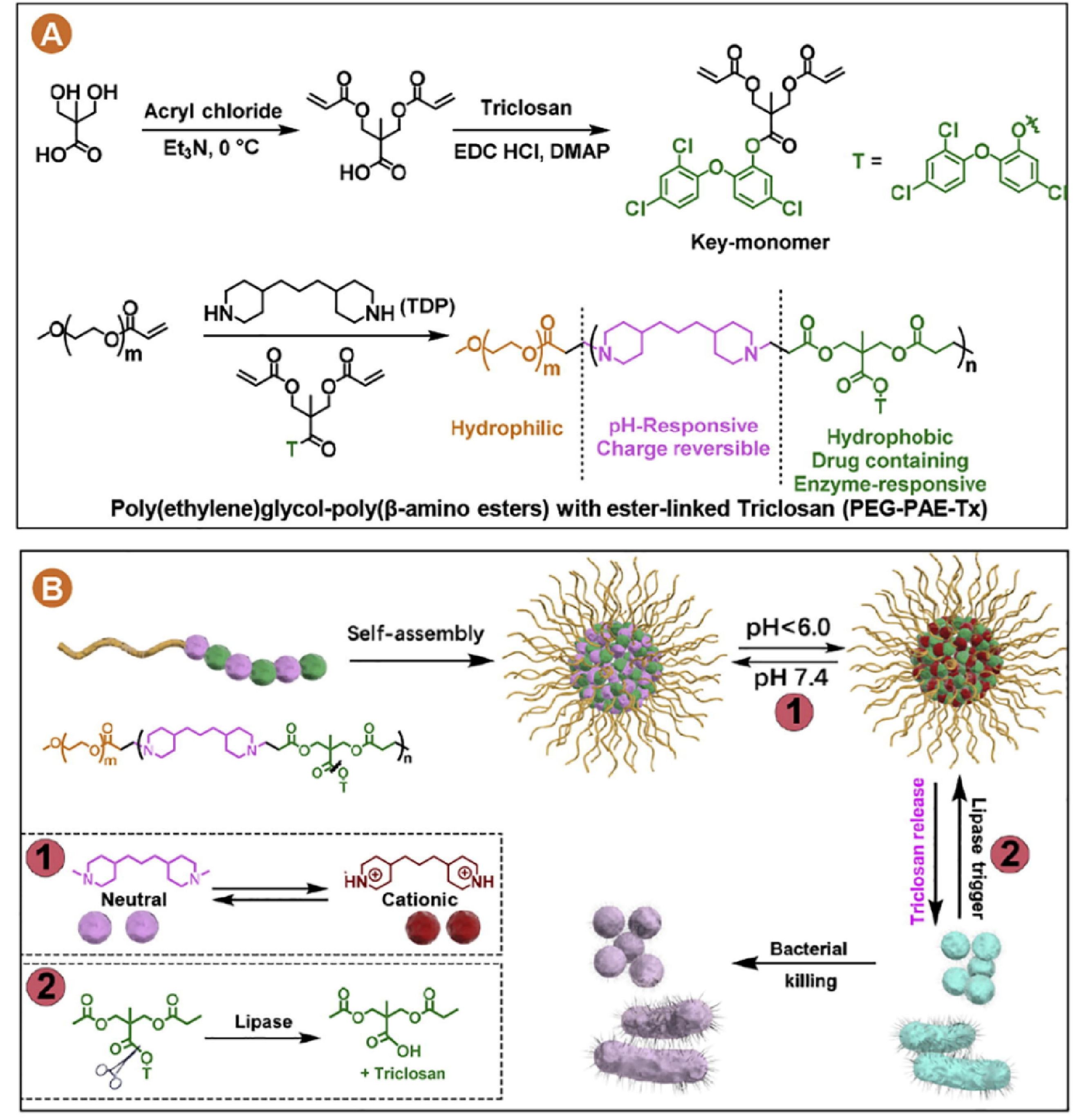
The above-mentioned antibiotics are loaded into nanocarriers expediently through hydrophobic interactions, which are weak and usually cannot prevent the drug premature leakage during storage and blood circulation.[98,99] Conjugating antibiotics in polymers, also named as polyprodrug, is a promising option to circumvent this problem. For instance, we conjugated triclosan onto a PEG-b-PAE block polymer via ester bonds. The triclosan-conjugated PEG-b-PAE forms stable polymeric micelles in water and possesses PEG shells that enable long blood circulation after being injected.[100] Triclosan is less likely to be released during blood circulation due to its chemical conjugation. While the PAE domain can be protonated at lower pH in a biofilm, facilitating the interactions between micelles and biofilm matrix and allowing the retention of micelles inside biofilms. The local bacterial lipases triggered the triclosan release to eradicate the biofilm-bacteria (Fig. 3).[100] PEG-PAE-Triclosan micelles could not only eradicate subcutaneous drug-resistant S. aureus infection in mice but also preferentially kill cariogenic oral pathogen S. mutans that secrete lipases in oral biofilms.
Loading hydrophilic antibiotics
Most of the commonly used antibiotics are hydrophilic because hydrophilic ones are more easily administered via oral or injection pathways. However, free hydrophilic antibiotics often suffer from shortcomings such as short blood retention, poor biodistribution, poor biofilm penetration, and potential side effects. To avoid these defects, diverse drug carriers such as hydrogels[101-109] and vesicles[110] have been developed. To be more specific, the focus of this section is the polymer-based delivery systems for hydrophilic antibiotics.
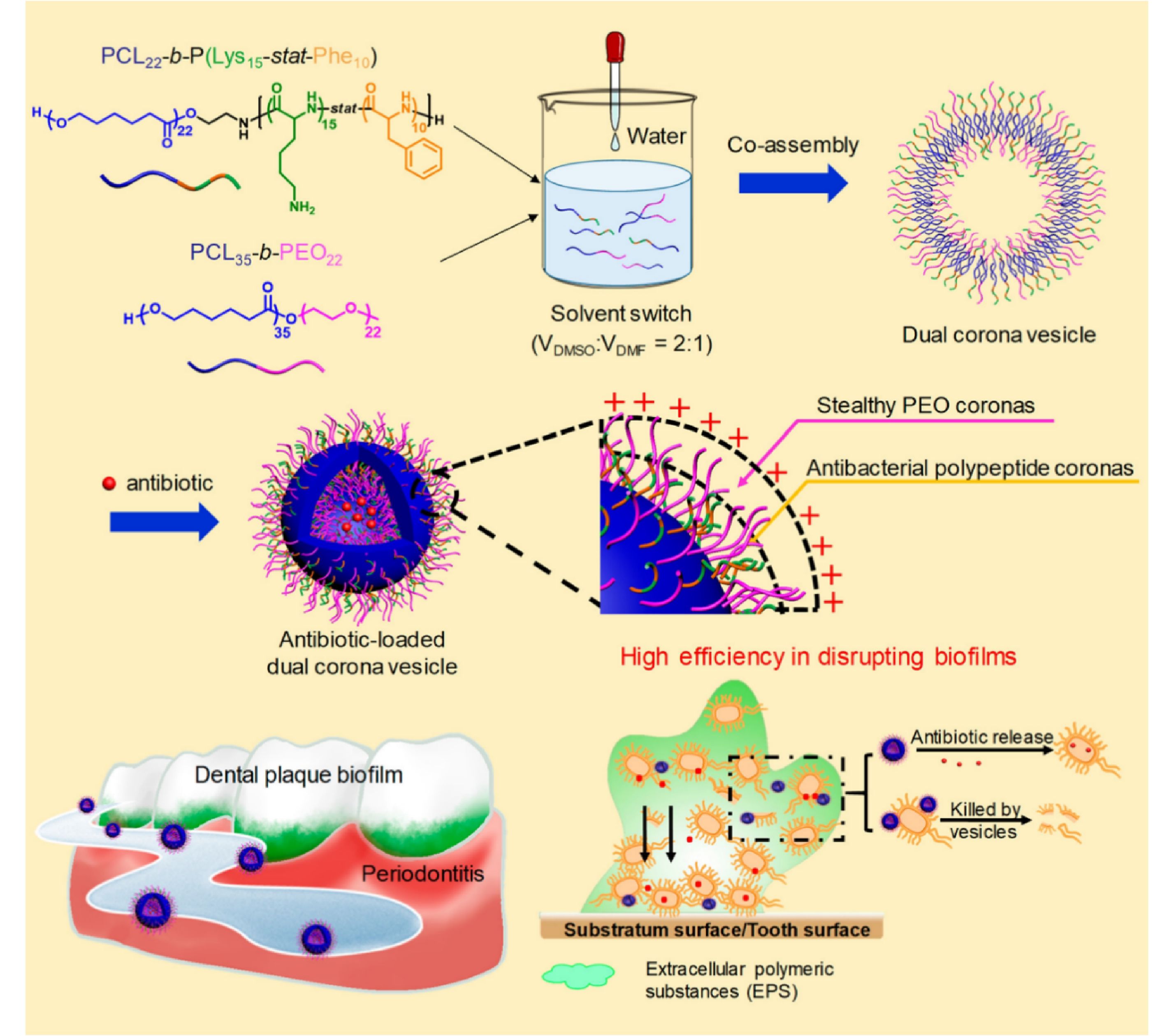
Among the polymer-based delivery systems for hydrophilic antibiotics, polymeric vesicles are of great importance. Just like polymeric micelles, polymeric vesicles are also assembled from amphiphilic copolymers. Notably, the difference is that the proportion of the hydrophobic domains in the amphiphilic copolymers used to construct polymeric vesicles is greater than that of copolymers used to build polymeric micelles. Compared to vesicles made of lipids, polymeric vesicles are usually more stable against the continuous dilution in the blood. Generally, the cores of the as-prepared polymeric vesicles are hydrophilic and the intermediate layers are hydrophobic. Thus, hydrophilic antibiotics are allowed to be loaded in the cores of polymeric vesicles, and hydrophobic antibiotics can be loaded in the intermediate layers at the same time. As a consequence, a great number of antibiotics could be loaded in vesicles, such as amikacin,[111] gentamicin,[111,112] tobramycin,[113-115] vancomycin,[116,117] azithromycin,[118] metronidazole,[119] oxacillin,[120] daptomycin,[121] doxycycline,[122] and antimicrobial peptides.[123] It is worth noting that the bilayer of vesicles can fuse with the structure of bacterial cell membranes, leading to plenty of antibiotics releasing once inside the biofilm.[110,124] However, different from the inhomogeneous features of bio-membranes, conventional synthetic polymer vesicles usually possess a homogeneous membrane, which significantly limited their versatility. Recently, Du et al. reviewed the recent advances and the future perspectives of design principles, synthesis, and biomedical applications of polymer vesicles with inhomogeneous membranes, which endow the vesicle with multi-functionality for various applications including drug delivery.[125] For example, Du et al. designed a multifunctional ciprofloxacin-loaded dual corona vesicle with intrinsic antibacterial activity for effective eradication of biofilm, which was made of two block copolymers, namely poly(ε-caprolactone)-block-poly(lysine-stat-phenylalanine) [PCL-b-P(Lys-stat-Phe)] and poly(ethylene oxide)-block-poly(ε-caprolactone) [PEO-b-PCL] (Fig. 4).[126] Similar to PEG, the stealthy property of PEO made vesicles repel the protein during blood circulation, and P(Lys-stat-Phe) enabied vesicles with positive charges, endowing them with targeting ability and bacterial membrane damaging ability.[126] After being encapsulated in vesicles, the dosage of ciprofloxacin could be reduced by 50% for the eradication of biofilms. Therefore, this work rendered an efficient approach to overcoming the biofilm barrier using polymeric vesicles.
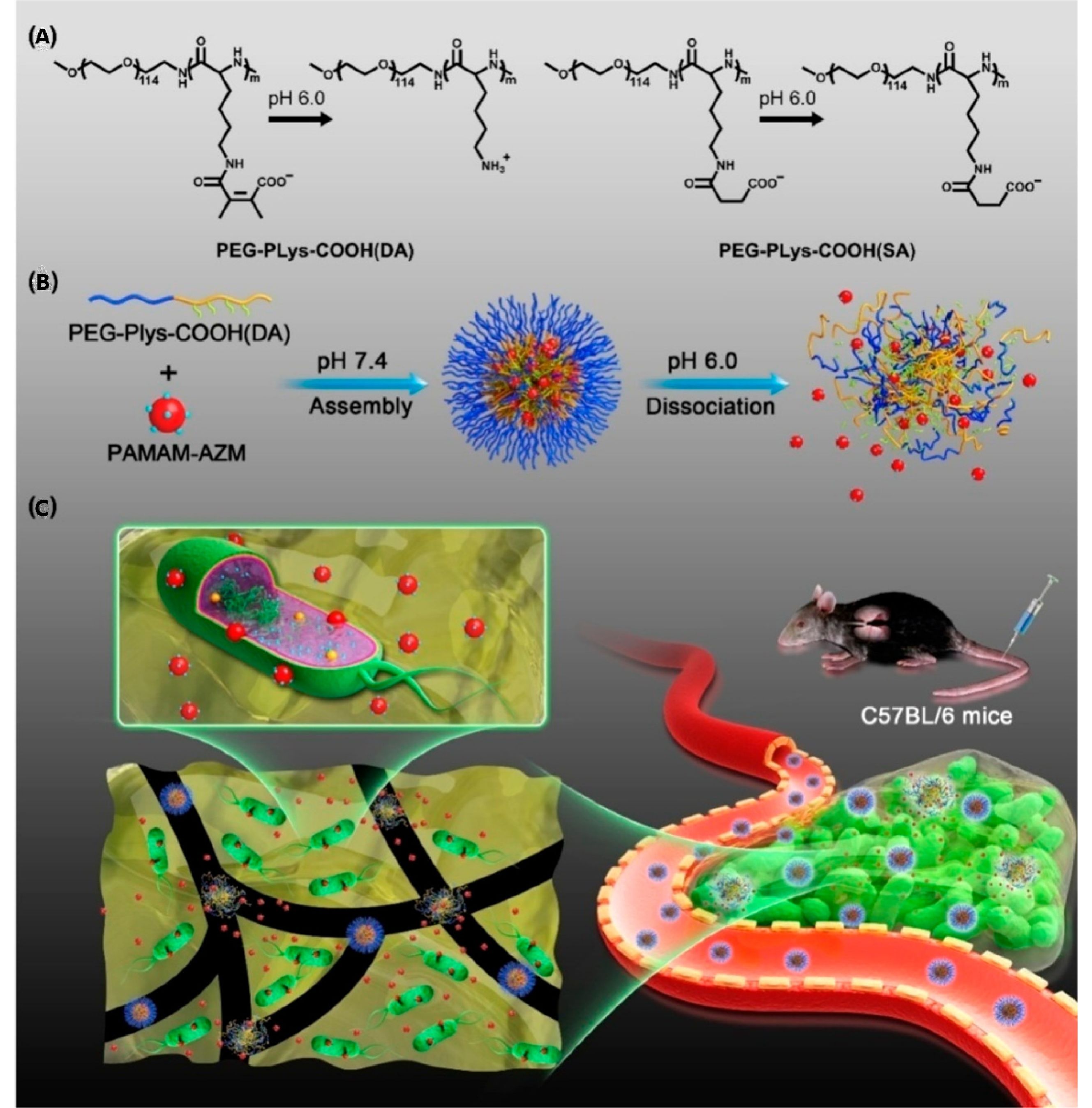
Besides, hydrophilic antimicrobials can be conjugated in polymers and applied as a prodrug as well. For example, Ji et al. conjugated azithromycin on amino-ended poly(amidoamine) dendrimer (PAMAM) as a prodrug (PAMAM-AZM NPs) and subsequently assembled them with 2,3-dimethyl maleic anhydride (DA) modified poly(ethylene glycol)-block-polylysine (PEG-b-PLys) to form nanoparticles (AZM-DA NPs) through electrostatic complexation (Fig. 5).[127] At low pH in a biofilm, the amide bond between PEG-b-Plys and DA is hydrolyzed and the positive net charge of the Plys domain is recovered, resulting in the dissociation of AZM-DA NPs and the release of PAMAM-AZM NPs. With a small size and positive charge, PAMAM-AZM NPs can penetrate and accumulate inside biofilms, exhibiting excellent antibiofilm activity. This research is promising for the treatment of biofilm-caused chronic infections.
Antimicrobial Nanosystems
Although many antibiotics delivery systems have been investigated and great progress has been made, polymeric systems with intrinsic bactericidal activities are still urgently needed. In nature, antimicrobial peptides (AMPs) exhibit excellent antibacterial activities.[128-133] Most AMPs are amphiphilic and possess cationic and hydrophobic moieties. Besides, they often possess an α-helical or β-sheet-like tubular secondary confirmation. Once interact with bacteria, the cationic moiety attracts negatively charged bacteria, and the hydrophobic moiety inserts into the lipophilic cell membrane domain, rupturing the cell membrane of bacteria.[134] This physical damage antibacterial mechanism is less likely to develop resistance.[135] Though AMPs are promising antimicrobials, low stability, high toxicity and expensive production limit their antibacterial application. To overcome these drawbacks, synthetic polymeric AMP mimics have been developed because of their facial synthesis with the key properties of AMPs.[22-25,135,136] Basically, these AMP mimics bearing positively charged domains such as primary amines, ammoniums, guanidiniums, thiazoliums, triazoliums, and phosphoniums on either main-chains or side-chains.[137] Also, they usually possess a rigid spatial configuration that regulates their bactericidal efficacy.[138] In certain cases, the secondary structures of the synthetic AMP mimics are transitionable under the trigger of stimuli. For example, Cheng et al. developed series of AMPs that could realize helix-coil conformation transition for regulating the antibacterial efficacy versus the cell lysis property of the polymers.[30,139,140] Moreover, synthetic AMP polymers can assemble into antimicrobials nanoparticles, thus increasing the local concentration of AMPs. Qiao et al. developed the synthesis of structurally nanoengineered antimicrobial peptide polymers (SNAPPs), which were unimolecular, forming star nanoparticles.[135] These SNAPPs showed superior antibacterial activity with several different antimicrobial mechanisms. Compared to the unimolecular AMPs, these nano-antimicrobials might be more stable during blood circulation and have greater biofilm penetration ability. Together with their multivalentcy effect, these nanoscale antimicrobials usually possess enhanced antibacterial activity.
Biofilm Dispersal
In a biofilm, the EPS matrix holds the embedded bacteria together like glue and limits the penetration of antibiotics. Therefore, dispersing the biofilm is another way to combat biofilm-related infections. In recent years, many kinds of molecular biofilm dispersants have been reported,[141-143] such as enzymes,[144-146] ROS,[147-150] nitric oxide (NO),[151] peptides,[152-155] molecules that regulate signaling pathways,[156,157] amino acids,[158] and biosurfactants.[159,160] But these molecular biofilm dispersants have disadvantages, limiting their applications. For example, DNase is easily inactivated by many factors, such as low pH and proteinases. Also, natural peptides are prone to be proteolyzed. Additionally, there were no beneficial effects of NO observed in vivo experiments because of the metabolic consumption.[161] The cytotoxicity of some dispersants has not been investigated. Fortunately, a combination of biofilm dispersants with nanoparticles can enhance their efficiency yet ameliorate their adverse effects.
After being loaded in polymer nanoparticles, the dispersants are protected in blood circulation,[162] and their stability is improved.[163] Also, with rational design, the loaded cargoes could be released in response to stimuli.[164-167] For example, when dispersion B was loaded with chitosan nanoparticles, the thermal stability and storage time were greatly improved.[168] Apart from encapsulation, the dispersants can be decorated on the surfaces of nanoparticles. For example, DNase I could be conjugated on the amino groups of chitosan nanoparticles.[169] Antimicrobials are often co-administered with these nano-dispersants to achieve desirable biofilm eradication efficacy. For example, ciprofloxacin was co-loaded into the DNase I-modified nanoparticles, and the simultaneous release of both DNase I and ciprofloxacin synergistically enhanced the biofilm eradication efficacy.
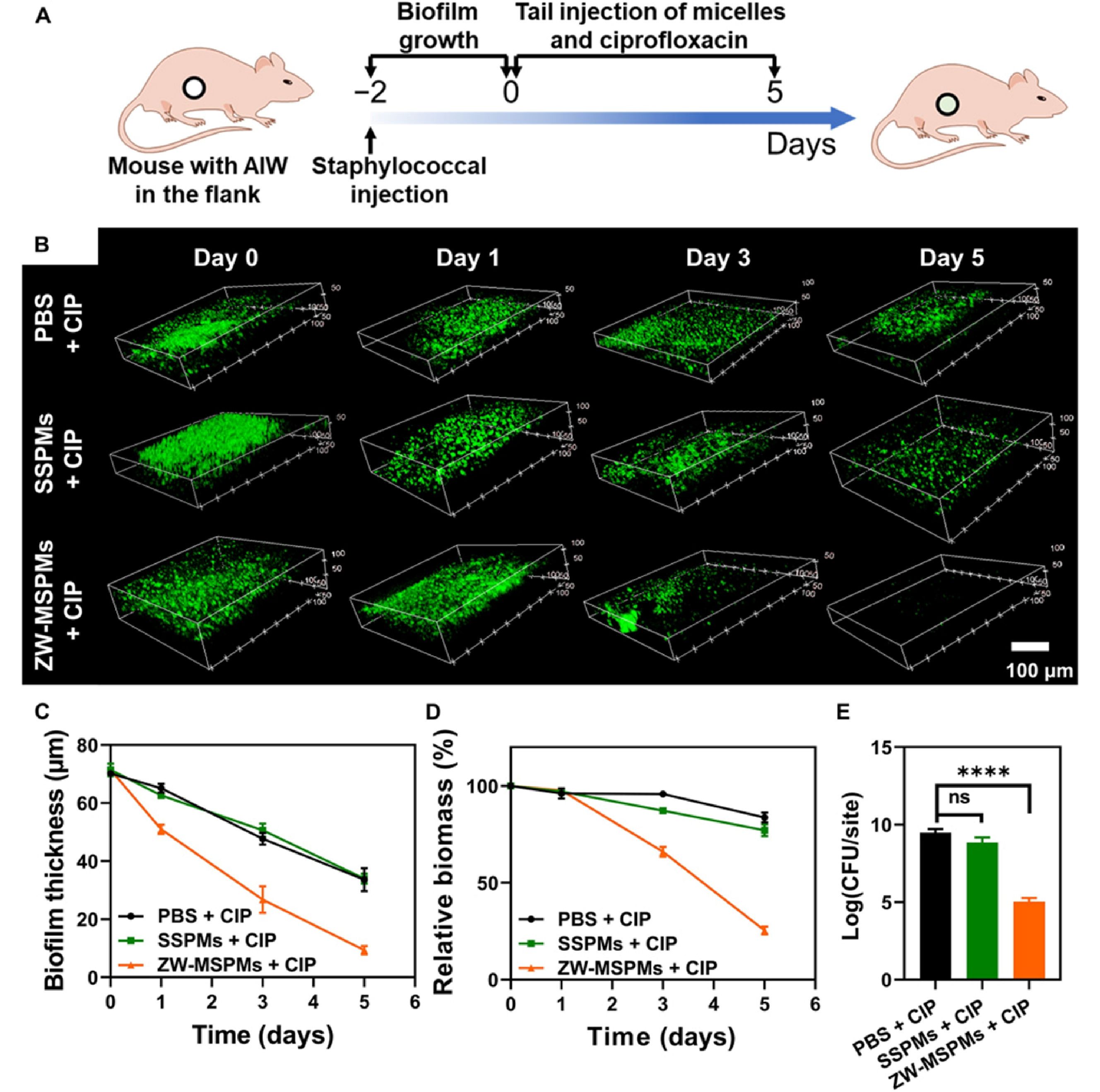
Besides, some polymer nanoparticles possess an intrinsic biofilm-dispersal property. Most of these biofilm-dispersal nanoparticles are cationic micelles or peptides because of their strong interactions with the negative bacteria and EPS.[170] However, the positive charge makes them easily cleared during blood circulation and has cytotoxicity to healthy cells.[171] Recently, we designed self-targeting zwitterionic MSPM (ZW-MSPM), which were self-assembled from PEG-b-PCL and poly(ε-caprolactone)-block-poly(quaternary-amino-ester) to address the above-mentioned issue.[172] Zwitterionic micelles are negatively charged at physiological conditions (pH 7.4) but transformed to a positive charge at low pH in a bacterial biofilm. This property endows the micelles with targeting ability in vivo. And the positive charge facilitates the micelles to penetrate and accumulate in biofilms, and subsequently disperse the biofilm. The in vivo efficacy of ZW-MSPM was observed in mice by growing staphylococcal biofilm underneath an intravital abdominal imaging window (AIW) (Fig. 6).[172]
To address the issue of delivering gaseous biofilm dispersants, Ji et al. developed a NO delivery system for the eradication of biofilms.[173] In this work, NO was conjugated on glutathione-responsive α-cyclodextrin (α-CD) as a prodrug, and integrated into a pH-sensitive block polypeptide copolymer together with α-CD-chlorin e6, forming supramolecular nanocarrier. Once inside biofilms, NO was rapidly released stimulated by the overexpressed glutathione in biofilms, which could synergistically eradicate biofilm with photodynamic therapy. This strategy may offer great possibilities for the eradication of biofilm-associated infections.
CONCLUSIONS AND FUTURE PERSPECTIVES
Biofilm-associated infections are posing a great threat to global human health. The demand and requirements for new antibiotic regimens remain largely unmet. The recent developments of polymer-based nanomaterials are of great potential in addressing the above-mentioned issues. Polymer-based nanosystems have been used to prevent bacterial adhesion and subsequent biofilm formation, as well as to eradicate the mature biofilms. Although polymer-based nanosystems possess numerous advantages, in our opinion, more efforts should be made on the following prospects:
(1) Polymer synthesis and characterizations. Until now, it is still quite challenging to synthesize polymers with exact molecular weights or with a narrow distribution. Besides, the synthesized polymers often differ in molecular weights and even chemical properties from batch to batch. Most of the synthesized polymers were characterized via nuclear magnetic resonance spectra and gel permeation chromatography measurements, etc, which, to some extent, are not enough to illustrate the chemical structures and properties due to the limitation of the apparatuses used. For example, the peaks of polymers in their NMR spectra are quite broad and the signal will be low if the polymers are not completely soluble.
(2) Currently, most of the polymeric nano-assemblies are formed by the driving forces of electrostatic attraction or hydrophobic interaction, which cannot fully prevent drug leakage during storage or circulation in the blood. Some strategies like cross-linking should be applied to reinforce the stability of nano-assemblies.[174]
(3) At present, most nanomaterials elucidated their antibacterial activity on biofilms that grow for 1 or 2 days. However, in clinical, the pathogenetic biofilms are formed over a long period with multiple bacterial species. Therefore, the anti-biofilm efficacy of the polymer-based materials should be evaluated on old biofilms that are more similar to real clinical situations in the future.
(4) Moreover, bacteria can also grow in mammalian cells. Most antibiotics cannot penetrate the mammalian cell membranes to eradicate these intracellular pathogens, causing chronic infections. Hence, nanosystems capable of targeting penetrate and across infected cell membranes should be taken into consideration. For example, our group recently have reported a macrophage membrane-coated antimicrobial nanoparticle, which could selectively enter into infected macrophages and wipe out the intracellular bacteria.[175]
(5) Despite many nanosystems have improved the efficiency of antibiotics, it is still at risk of developing drug resistance. Future infection control strategies should concentrate more on non-antibiotic-containing systems, such as photodynamic therapy, photothermal therapy, and systems based on cascade reactions.[176]
BIOGRAPHY
Yong Liu obtained his PhD degree from Nankai University, Tianjin, China, in 2016 under the supervision of Prof. Linqi Shi, majoring in polymer chemistry and physics. After that, he joined the Department of Biomedical Engineering at the University Medical Center Groningen/University of Groningen, the Netherlands, to work on novel nanotechnology-based infection control strategies. In 2019, he was an associate professor at FUNSOM, Soochow University. He joined Wenzhou Institute, University of Chinese Academy of Sciences in 2021. Currently, his research interest is focused on using polymer-based materials to control the emergence of multidrug resistance in microbes.
Lin-Qi Shi received his PhD from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, China (1993). He is currently a full professor at the Institute of Polymer Chemistry, Nankai University, Tianjin, China. His research involves polymerization, self-assembly of block copolymers and polymeric nanocarriers for biomedical applications. He has over 240 peer-reviewed publications in polymer science and has been awarded an Excellent Professor Award by the Ministry of Education, China (2002). He was one of the Distinguished Young Scholars financed by the National Natural Science Foundation of China (2006).
Antimicrobial resistance: a global challenge
Sci. Transl. Med. 2014 6 236ed10Howard, S. J.; Hopwood, S.; Davies, S. C. Antimicrobial resistance: a global challenge. Sci. Transl. Med. 2014, 6, 236ed10.
Understanding biofilm resistance to antibacterial agents
Nat. Rev. Drug Discov. 2003 2 114 122Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114−122.
Bacterial biofilms: from the natural environment to infectious diseases
Nat. Rev. Microbiol. 2004 2 95 108Hall-Stoodley, L.; Costerton, J. W.; Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95−108.
The biofilm matrix
Nat. Rev. Microbiol. 2010 8 623 633Flemming, H. C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623−633.
The role of efflux and physiological adaptation in biofilm tolerance and resistance
J. Biol. Chem. 2016 291 12565 12572Van Acker, H.; Coenye, T. The role of efflux and physiological adaptation in biofilm tolerance and resistance. J. Biol. Chem. 2016, 291, 12565−12572.
Aminoglycoside modifying enzymes
Drug Resistance Update. 2010 13 151 171Ramirez, M. S.; Tolmasky, M. E. Aminoglycoside modifying enzymes. Drug Resistance Update. 2010, 13, 151−171.
Antibiotic resistance of bacterial biofilms
Int. J. Antimicrob. Agents. 2010 35 322 332Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010, 35, 322−332.
The crisis of no new antibiotics-what is the way forward?
Lancet Infect. Dis. 2012 12 249 253Piddock, L. J. V. The crisis of no new antibiotics-what is the way forward? Lancet Infect. Dis. 2012, 12, 249−253.
Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control
Chem. Soc. Rev. 2019 48 428 446Liu, Y.; Shi, L.; Su, L.; van der Mei, H. C.; Jutte, P. C.; Ren, Y.; Busscher, H. J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428−446.
Recent advances in self-assembled nano-therapeutics
Chinese J. Polym. Sci. 2018 36 322 346Zheng, C, X.; Zhao, Y.; Liu, Y. Recent advances in self-assembled nano-therapeutics. Chinese J. Polym. Sci. 2018, 36, 322−346.
Macromolecular engineering by atom transfer radical polymerization
J. Am. Chem. Soc. 2014 136 6513 6533Matyjaszewski, K.; Tsarevsky, N. V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513−6533.
Polymer vectors via controlled/living radical polymerization for gene delivery
Prog. Polym. Sci. 2011 36 1099 1131Xu, F. J.; Yang, W. T. Polymer vectors via controlled/living radical polymerization for gene delivery. Prog. Polym. Sci. 2011, 36, 1099−1131.
Stereoblock copolymers and tacticity control in controlled/living radical polymerization
J. Am. Chem. Soc. 2003 125 6986 6993Lutz, J. F.; Neugebauer, D.; Matyjaszewski, K. Stereoblock copolymers and tacticity control in controlled/living radical polymerization. J. Am. Chem. Soc. 2003, 125, 6986−6993.
Facile synthesis of high molecular weight polypeptides via fast and moisture insensitive polymerization of α-amino acid n-carboxyanhydrides
Chinese J. Polym. Sci. 2020 38 1131 1140Wu, Y. M.; Zhang, W. W.; Zhou, R. Y.; Chen, Q.; Xie, C. Y.; Xiang, H. X.; Sun, B.; Zhu, M. F.; Liu, R. H. Facile synthesis of high molecular weight polypeptides via fast and moisture insensitive polymerization of α-amino acid n-carboxyanhydrides. Chinese J. Polym. Sci. 2020, 38, 1131−1140.
Biodegradable antibacterial polymeric nanosystems: a new hope to cope with multidrug-resistant bacteria
Small 2019 15 e1900999Ding, X.; Wang, A.; Tong, W.; Xu, F. J. Biodegradable antibacterial polymeric nanosystems: a new hope to cope with multidrug-resistant bacteria. Small 2019, 15, e1900999.
Biodegradable broad-spectrum antimicrobial polycarbonates: investigating the role of chemical structure on activity and selectivity
Macromolecules 2013 46 8797 8807Chin, W.; Yang, C. A.; Ng, V. W. L.; Huang, Y.; Cheng, J. C.; Tong, Y. W.; Coady, D. J.; Fan, W. M.; Hedrick, J. L.; Yang, Y. Y. Biodegradable broad-spectrum antimicrobial polycarbonates: investigating the role of chemical structure on activity and selectivity. Macromolecules 2013, 46, 8797−8807.
Engineered polymer nanoparticles with unprecedented antimicrobial efficacy and therapeutic indices against multidrug-resistant bacteria and biofilms
J. Am. Chem. Soc. 2018 140 12137 12143Gupta, A.; Landis, R. F.; Li, C. H.; Schnurr, M.; Das, R.; Lee, Y. W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V. M. Engineered polymer nanoparticles with unprecedented antimicrobial efficacy and therapeutic indices against multidrug-resistant bacteria and biofilms. J. Am. Chem. Soc. 2018, 140, 12137−12143.
Synthesis and antibacterial study of sulfobetaine/quaternary ammonium-modified star-shaped poly[2-(dimethylamino)ethyl methacrylate]-based copolymers with an inorganic core
Biomacromolecules 2017 18 44 55Pu, Y. J.; Hou, Z.; Khin, M. M.; Zamudio-Vazquez, R.; Poon, K. L.; Duan, H. W.; Chan-Park, M. B. Synthesis and antibacterial study of sulfobetaine/quaternary ammonium-modified star-shaped poly[2-(dimethylamino)ethyl methacrylate]-based copolymers with an inorganic core. Biomacromolecules 2017, 18, 44−55.
Broad spectrum macromolecular antimicrobials with biofilm disruption capability and in vivo efficacy
Adv. Healthc. Mater. 2017 6 1601420Tan, J. P. K.; Coady, D. J.; Sardon, H.; Yuen, A.; Gao, S. J.; Lim, S. W.; Liang, Z. C.; Tan, E. W.; Venkataraman, S.; Engler, A. C.; Fevre, M.; Ono, R.; Yang, Y. Y.; Hedrick, J. L. Broad spectrum macromolecular antimicrobials with biofilm disruption capability and in vivo efficacy. Adv. Healthc. Mater. 2017, 6, 1601420.
Antibacterial behaviour of quaternized poly(vinyl chloride)-g-poly(4-vinyl pyridine) graft copolymers
Chinese J. Polym. Sci. 2015 33 265 274Patel, M.; Patel, R.; Chi, W. S.; Kim, J. H.; Sung, J. S. Antibacterial behaviour of quaternized poly(vinyl chloride)-g-poly(4-vinyl pyridine) graft copolymers. Chinese J. Polym. Sci. 2015, 33, 265−274.
Multifunctional biocompatible and biodegradable folic acid conjugated poly(ε-caprolactone)-polypeptide copolymer vesicles with excellent antibacterial activities
Bioconjug. Chem. 2015 26 725 734Wang, M.; Zhou, C.; Chen, J.; Xiao, Y.; Du, J. Multifunctional biocompatible and biodegradable folic acid conjugated poly(ε-caprolactone)-polypeptide copolymer vesicles with excellent antibacterial activities. Bioconjug. Chem. 2015, 26, 725−734.
Preparation and antibacterial mechanism insight of polypeptide-based micelles with excellent antibacterial activities
Biomacromolecules 2016 17 3922 3930Xi, Y.; Song, T.; Tang, S.; Wang, N.; Du, J. Preparation and antibacterial mechanism insight of polypeptide-based micelles with excellent antibacterial activities. Biomacromolecules 2016, 17, 3922−3930.
Cationic amphiphilic polymers with antimicrobial activity for oral care applications: eradication of S. mutans biofilm
Biomacromolecules 2017 18 257 265Takahashi, H.; Nadres, E. T.; Kuroda, K. Cationic amphiphilic polymers with antimicrobial activity for oral care applications: eradication of S. mutans biofilm. Biomacromolecules 2017, 18, 257−265.
Polycarbonates with potent and selective antimicrobial activity toward gram-positive bacteria
Biomacromolecules 2017 18 87 95Nimmagadda, A.; Liu, X.; Teng, P.; Su, M.; Li, Y.; Qiao, Q.; Khadka, N. K.; Sun, X.; Pan, J.; Xu, H.; Li, Q.; Cai, J. Polycarbonates with potent and selective antimicrobial activity toward gram-positive bacteria. Biomacromolecules 2017, 18, 87−95.
ε-Poly(L-lysine)-based hydrogels with fast-acting and prolonged antibacterial activities
Chinese J. Polym. Sci. 2018 36 1239 1250Zou, Y. J.; He, S. S.; Du, J. Z. ε-Poly(L-lysine)-based hydrogels with fast-acting and prolonged antibacterial activities. Chinese J. Polym. Sci. 2018, 36, 1239−1250.
Antimicrobial properties and application of polysaccharides and their derivatives
Chinese J. Polym. Sci. 2021 39 133 146Xia, G. X.; Wu, Y. M.; Bi, Y. F.; Chen, K.; Zhang, W. W.; Liu, S. Q.; Zhang, W. J.; Liu, R. H. Antimicrobial properties and application of polysaccharides and their derivatives. Chinese J. Polym. Sci. 2021, 39, 133−146.
A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action
J. Biol. Chem. 2001 276 43767 43774Ibrahim, H. R.; Thomas, U.;Pellegrini, A. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 2001, 276, 43767−43774.
pH-Switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds
ACS Nano 2019 13 11686 11697Wang, J.; Chen, X. Y.; Zhao, Y.; Yang, Y.; Wang, W.; Wu, C.; Yang, B.; Zhang, Z.; Zhang, L.; Liu, Y.; Du, X.; Li, W.; Qiu, L.; Jiang, P.; Mou, X. Z.;Li, Y. Q. pH-Switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano 2019, 13, 11686−11697.
Helical antimicrobial polypeptides with radial amphiphilicity
Proc. Natl. Acad. Sci. U. S. A. 2015 112 13155 13160Xiong, M.; Lee, M. W.; Mansbach, R. A.; Song, Z.; Bao, Y.; Peek, R. M.; Yao, C.; Chen, L. F.; Ferguson, A. L.; Wong, G. C. L.; Cheng, J. J. Helical antimicrobial polypeptides with radial amphiphilicity. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 13155−13160.
Controlled drug delivery systems in eradicating bacterial biofilm-associated infections
J. Control. Release 2021 329 1102 1116Liu, Y.; Li, Y.; Shi, L. Controlled drug delivery systems in eradicating bacterial biofilm-associated infections. J. Control. Release 2021, 329, 1102−1116.
A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs
Cancer Res. 1986 46 6387 6392Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387−6392.
Cancer nanomedicine: progress, challenges and opportunities
Nat. Rev. Cancer 2017 17 20 37Shi, J.; Kantoff, P. W.; Wooster, R.; Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20−37.
Microvascular permeability of normal and neoplastic tissues
Microvasc. Res. 1986 31 288 305Gerlowski, L. E.; Jain, R. K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 1986, 31, 288−305.
The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting
Adv. Enzyme Regul. 2001 41 189 207Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189−207.
Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy
Nano Today 2020 35 100970Ding, Y.; Xu, Y.; Yang, W.; Niu, P.; Li, X.; Chen, Y.; Li, Z.; Liu, Y.; An, Y.; Liu, Y.; Shen, W.; Shi, L. Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy. Nano Today 2020, 35, 100970.
The enhanced permeability and retention effect based nanomedicine at the site of injury
Nano Res. 2020 13 564 569Liu, Y.; Sun, D.; Fan, Q.; Ma, Q.; Dong, Z.; Tao, W.; Tao, H.; Liu, Z.; Wang, C. The enhanced permeability and retention effect based nanomedicine at the site of injury. Nano Res. 2020, 13, 564−569.
The 35th anniversary of the discovery of EPR effect: a new wave of nanomedicines for tumor-targeted drug delivery-personal remarks and future prospects
J. Pers Med. 2021 11 229Maeda, H. The 35th anniversary of the discovery of EPR effect: a new wave of nanomedicines for tumor-targeted drug delivery-personal remarks and future prospects. J. Pers Med. 2021, 11, 229.
Effect of microbial and mite proteases on low and high molecular weight kininogens. Generation of kinin and inactivation of thiol protease inhibitory activity
J. Biol. Chem. 1993 268 17711 17715Maruo, K.; Akaike, T.; Inada, Y.; Ohkubo, I.; Ono, T.; Maeda, H. Effect of microbial and mite proteases on low and high molecular weight kininogens. Generation of kinin and inactivation of thiol protease inhibitory activity. J. Biol. Chem. 1993, 268, 17711−17715.
Activation of hageman factor and prekallikrein and generation of kinin by various microbial proteinases
J. Biol. Chem. 1989 264 10589 10594Molla, A.; Yamamoto, T.; Akaike, T.; Miyoshi, S.; Maeda, H. Activation of hageman factor and prekallikrein and generation of kinin by various microbial proteinases. J. Biol. Chem. 1989, 264, 10589−10594.
A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pigs
Infect. Immun. 1985 48 747 753Kamata, R.; Yamamoto, T.; Matsumoto, K.; Maeda, H. A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pigs. Infect. Immun. 1985, 48, 747−753.
Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease
J. Biochem. 1984 96 739 749Matsumoto, K.; Yamamoto, T.; Kamata, R.; Maeda, H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J. Biochem. 1984, 96, 739−749.
Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine
Appl. Environ. Microbiol. 2010 76 2326 2334von Ohle, C.; Gieseke, A.; Nistico, L.; Decker, E. M.; deBeer, D.; Stoodley, P. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Appl. Environ. Microbiol. 2010, 76, 2326−2334.
Analysis of pH, pO2 and pCO2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgery
Infection 1994 22 386 389Simmen, H. P.; Battaglia, H.; Giovanoli, P.; Blaser, J. Analysis of pH, pO2 and pCO2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgery. Infection 1994, 22, 386−389.
Antifungal-inbuilt metal-organic-frameworks eradicate Candida albicans biofilms
Adv. Funct. Mater. 2020 30 2000537Su, L.; Li, Y.; Liu, Y.; Ma, R.; Liu, Y.; Huang, F.; An, Y.; Ren, Y.; van der Mei, H. C.; Busscher, H. J.; Shi, L. Antifungal-inbuilt metal-organic-frameworks eradicate Candida albicans biofilms. Adv. Funct. Mater. 2020, 30, 2000537.
Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery
Am. J. Surg. 1993 166 24 27Simmen, H. P.; Blaser, J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am. J. Surg. 1993, 166, 24−27.
Recent advances and future prospects on adaptive biomaterials for antimicrobial applications
Macromol. Biosci. 2019 19 1900289Su, L.; Li, Y.; Liu, Y.; An, Y.; Shi, L. Recent advances and future prospects on adaptive biomaterials for antimicrobial applications. Macromol. Biosci. 2019, 19, 1900289.
Physiological heterogeneity in biofilms
Nat. Rev. Microbiol. 2008 6 199 210Stewart, P. S.; Franklin, M. J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199−210.
Biofilms: an emergent form of bacterial life
Nat. Rev. Microbiol. 2016 14 563 575Flemming, H. C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S. A.; Kjelleberg, S. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563−575.
Hypoxia-responsive nanocarriers for cancer imaging and therapy: recent approaches and future perspectives
Chem. Commun. 2016 52 8492 8500Thambi, T.; Park, J. H.; Lee, D. S. Hypoxia-responsive nanocarriers for cancer imaging and therapy: recent approaches and future perspectives. Chem. Commun. 2016, 52, 8492−8500.
Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review
J. Control. Release 2020 319 135 156Kumari, R.; Sunil, D.; Ningthoujam, R. S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review. J. Control. Release 2020, 319, 135−156.
Relief of biofilm hypoxia using an oxygen nanocarrier: a new paradigm for enhanced antibiotic therapy
Adv. Sci. 2020 7 2000398Hu, D.; Zou, L.; Yu, W.; Jia, F.; Han, H.; Yao, K.; Jin, Q.; Ji, J. Relief of biofilm hypoxia using an oxygen nanocarrier: a new paradigm for enhanced antibiotic therapy. Adv. Sci. 2020, 7, 2000398.
Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents-An in-depth review of the last two decades
WIREs-Nanomed. Nanobiotechnol. 2021 13 e1664Devnarain, N.; Osman, N.; Fasiku, V. O.; Makhathini, S.; Salih, M.; Ibrahim, U. H.; Govender, T. Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents-An in-depth review of the last two decades. WIREs-Nanomed. Nanobiotechnol. 2021, 13, e1664.
The first generation of β-galactosidase-responsive prodrugs designed for the selective treatment of solid tumors in prodrug monotherapy
Angew. Chem. Int. Ed. 2012 51 11606 11610Legigan, T.; Clarhaut, J.; Tranoy-Opalinski, I.; Monvoisin, A.; Renoux, B.; Thomas, M.; Le Pape, A.; Lerondel, S.; Papot, S. The first generation of β-galactosidase-responsive prodrugs designed for the selective treatment of solid tumors in prodrug monotherapy. Angew. Chem. Int. Ed. 2012, 51, 11606−11610.
Phosphatases and kinases delivered to the host cell by bacterial pathogens
Trends Microbiol. 2000 8 29 33DeVinney, R.; Steele-Mortimer, O.; Finlay, B. B. Phosphatases and kinases delivered to the host cell by bacterial pathogens. Trends Microbiol. 2000, 8, 29−33.
Real time detection of ESKAPE pathogens by a nitroreductase-triggered fluorescence turn-on probe
Chem. Commun. 2017 53 11177 11180Xu, S.; Wang, Q.; Zhang, Q.; Zhang, L.; Zuo, L.; Jiang, J. D.; Hu, H. Y. Real time detection of ESKAPE pathogens by a nitroreductase-triggered fluorescence turn-on probe. Chem. Commun. 2017, 53, 11177−11180.
Dispersal of bap-mediated Staphylococcus aureus biofilm by proteinase K
J. Antibiotics 2013 66 55 60Kumar Shukla, S.; Rao, T. S. Dispersal of bap-mediated Staphylococcus aureus biofilm by proteinase K. J. Antibiotics 2013, 66, 55−60.
Bacterial lipases: an overview of production, purification and biochemical properties
Appl. Microbiol. Biotechnol. 2004 64 763 781Gupta, R.; Gupta, N.; Rathi, P. Bacterial lipases: an overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol. 2004, 64, 763−781.
Bacterial phospholipases and their role in virulence
Trends Microbiol. 1997 5 156 161Songer, J. G. Bacterial phospholipases and their role in virulence. Trends Microbiol. 1997, 5, 156−161.
Bacterial toxins: a table of lethal amounts
Microbiol. Rev. 1982 46 86 94Gill, D. M. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 1982, 46, 86−94.
Bacterial toxins: cellular mechanisms of action
Microbiol. Rev. 1984 48 199 221Middlebrook, J. L.; Dorland, R. B. Bacterial toxins: cellular mechanisms of action. Microbiol. Rev. 1984, 48, 199−221.
Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents
Angew. Chem. In. Ed. 2016 55 1760 1764Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew. Chem. In. Ed. 2016, 55, 1760−1764.
Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo
J. Immunol. 2001 167 5067 5076Pulendran, B.; Kumar, P.; Cutler, C. W.; Mohamadzadeh, M.; Van Dyke, T.; Banchereau, J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 2001, 167, 5067−5076.
Unraveling the biological roles of reactive oxygen species
Cell Metab. 2011 13 361 366Murphy, M. P.; Holmgren, A.; Larsson, N. G.; Halliwell, B.; Chang, C. J.; Kalyanaraman, B.; Rhee, S. G.; Thornalley, P. J.; Partridge, L.; Gems, D.; Nystrom, T.; Belousov, V.; Schumacker, P. T.; Winterbourn, C. C. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361−366.
Recent advances on reactive oxygen species-responsive delivery and diagnosis system
Biomacromolecules. 2019 20 2441 2463Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent advances on reactive oxygen species-responsive delivery and diagnosis system. Biomacromolecules. 2019, 20, 2441−2463.
Glucose and H2O2 dual-sensitive nanogels for enhanced glucose-responsive insulin delivery
Nanoscale 2019 11 9163 9175Li, C.; Liu, X.; Liu, Y.; Huang, F.; Wu, G.; Liu, Y.; Zhang, Z.; Ding, Y.; Lv, J.; Ma, R.; An, Y.; Shi, L. Glucose and H2O2 dual-sensitive nanogels for enhanced glucose-responsive insulin delivery. Nanoscale 2019, 11, 9163−9175.
Photodynamic therapy-triggered on-demand drug release from ROS-responsive core-cross-linked micelles toward synergistic anti-cancer treatment
Nano Res. 2019 12 999 1008Li, Y.; Hu, J.; Liu, X.; Liu, Y.; Lv, S.; Dang, J.; Ji, Y.; He, J.; Yin, L. Photodynamic therapy-triggered on-demand drug release from ROS-responsive core-cross-linked micelles toward synergistic anti-cancer treatment. Nano Res. 2019, 12, 999−1008.
Bioinspired antifouling polymers
Mater. Today 2005 8 38 46Dalsin, J. L.; Messersmith, P. B. Bioinspired antifouling polymers. Mater. Today 2005, 8, 38−46.
Doubly biomimetic catecholic phosphorylcholine copolymer: a platform strategy for fabricating antifouling surfaces
Macromol. Biosci. 2012 12 979 985Gong, Y. K.; Liu, L. P.; Messersmith, P. B. Doubly biomimetic catecholic phosphorylcholine copolymer: a platform strategy for fabricating antifouling surfaces. Macromol. Biosci. 2012, 12, 979−985.
Reversibly switching the function of a surface between attacking and defending against bacteria
Angew. Chem. Int. Ed. 2012 51 2602 2605Cao, Z.; Mi, L.; Mendiola, J.; Ella-Menye, J. R.; Zhang, L.; Xue, H.; Jiang, S. Reversibly switching the function of a surface between attacking and defending against bacteria. Angew. Chem. Int. Ed. 2012, 51, 2602−2605.
Novel infection-resistant surface coatings: a bioengineering approach
MRS Bull. 2011 36 357 366Khoo, X.; Grinstaff, M. W. Novel infection-resistant surface coatings: a bioengineering approach. MRS Bull. 2011, 36, 357−366.
Bacterial and mammalian cell response to poly(3-sulfopropyl methacrylate) brushes loaded with silver halide salts
Biomaterials 2009 30 1524 1531Ramstedt, M.; Ekstrand-Hammarstrom, B.; Shchukarev, A. V.; Bucht, A.; Osterlund, L.; Welch, M.; Huck, W. T. S. Bacterial and mammalian cell response to poly(3-sulfopropyl methacrylate) brushes loaded with silver halide salts. Biomaterials 2009, 30, 1524−1531.
Development of nanoparticles for antimicrobial drug delivery
Curr. Med. Chem. 2010 17 585 594Zhang, L.; Pornpattananangkul, D.; Hu, C. M. J.; Huang, C. M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585−594.
Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins)
Biochim. Biophys. Acta-Biomembr. 2007 1768 2500 2509Epand, R. F.; Savage, P. B.; Epand, R. M. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins). Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2500−2509.
Construction of a densely poly(ethylene glycol)-chain-tethered surface and its performance
Polym. J. 2011 43 949 958Nagasaki, Y. Construction of a densely poly(ethylene glycol)-chain-tethered surface and its performance. Polym. J. 2011, 43, 949−958.
Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications
Adv. Mater. 2010 22 920 932Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920−932.
pH responsive properties of non-fouling mixed-charge polymer brushes based on quaternary amine and carboxylic acid monomers
Biomaterials 2010 31 2919 2925Mi, L.; Bernards, M. T.; Cheng, G.; Yu, Q.; Jiang, S. pH responsive properties of non-fouling mixed-charge polymer brushes based on quaternary amine and carboxylic acid monomers. Biomaterials 2010, 31, 2919−2925.
Manipulating sticky and non-sticky properties in a single material
Angew. Chem. Int. Ed. 2011 50 6102 6104Cao, Z.; Brault, N.; Xue, H.; Keefe, A.; Jiang, S. Manipulating sticky and non-sticky properties in a single material. Angew. Chem. Int. Ed. 2011, 50, 6102−6104.
Micro- and nanotopography sensitive bacterial attachment mechanisms: a review
Front. Microbiol. 2019 10 191Cheng, Y.; Feng, G.; Moraru, C. I. Micro- and nanotopography sensitive bacterial attachment mechanisms: a review. Front. Microbiol. 2019, 10, 191.
How microbes read the map: effects of implant topography on bacterial adhesion and biofilm formation
Biomaterials 2021 268 120595Lee, S. W.; Phillips, K. S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595.
Nanogels: a novel approach in antimicrobial delivery systems and antimicrobial coatings
Bioactive Mater. 2021 6 3634 3657Keskin, D.; Zu, G.; Forson, A. M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: a novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioactive Mater. 2021, 6, 3634−3657.
Inhibiting bacterial adhesion by mechanically modulated microgel coatings
Biomacromolecules 2019 20 243 253Keskin, D.; Mergel, O.; van der Mei, H. C.; Busscher, H. J.; van Rijn, P. Inhibiting bacterial adhesion by mechanically modulated microgel coatings. Biomacromolecules 2019, 20, 243−253.
A biomimetic surface for infection-resistance through assembly of metal-phenolic networks
Chinese J. Polym. Sci. 2018 36 576 583Jiang, R. J.; Yan, S. J.; Tian, L. M.; Xu, S. A.; Xin, Z. R.; Luan, S. F.; Yin, J. H.; Ren, L. Q.; Zhao, J. A biomimetic surface for infection-resistance through assembly of metal-phenolic networks. Chinese J. Polym. Sci. 2018, 36, 576−583.
Self-adaptive antibacterial porous implants with sustainable responses for infected bone defect therapy
Adv. Funct. Mater. 2019 29 1807915Jin, X.; Xiong, Y. H.; Zhang, X. Y.; Wang, R.; Xing, Y.; Duan, S.; Chen, D.; Tian, W.; Xu, F. J. Self-adaptive antibacterial porous implants with sustainable responses for infected bone defect therapy. Adv. Funct. Mater. 2019, 29, 1807915.
The self-assembly of polystyrene-b-poly(acrylic acid)/polystyrene in water
Acta Polymerica Sinica (in Chinese) 2005 379 383Zhao, C. J.; An, Y. L.; Yin, F. F.; Zhang, W. Q.; Shi, L. Q. The self-assembly of polystyrene-b-poly(acrylic acid)/polystyrene in water. Acta Polymerica Sinica (in Chinese) 2005, 379−383.
Investigation of the micellization process of diblock copolymers containing pH sensitive poly(4-vinylpyridine) by NMR
Acta Polymerica Sinica (in Chinese) 2008 691 696He, Z.; Sun, P.; Xiong, D. A.; Ma, R.; Lin, H.; Shi, L. Investigation of the micellization process of diblock copolymers containing pH sensitive poly(4-vinylpyridine) by NMR. Acta Polymerica Sinica (in Chinese) 2008, 691−696.
Recent progress in biomimetic light-harvesting materials
Acta Polymerica Sinica (in Chinese) 2012 1108 1117Chai, Z. H.; Ma, R. J.; Zhang, Z. K.; Shi, L. Q. Recent progress in biomimetic light-harvesting materials. Acta Polymerica Sinica (in Chinese) 2012, 1108−1117.
Solution behavior of comb-like copolymer dispersants probed by laser light scattering
Acta Polymerica Sinica (in Chinese) 2013 750 754Xiong, J.; Wang, J. Z.; Ran, Q. P.; An, Y. L.; Zhang, Z. K.; Shi, L. Q. Solution behavior of comb-like copolymer dispersants probed by laser light scattering. Acta Polymerica Sinica (in Chinese) 2013, 750−754.
Tpps aggregates with chirality induced by hydrogen bonds from polycationic glycoconjugate
Acta Polymerica Sinica (in Chinese) 2014 1378 1385Hao, J.; Li, A.; Liu, Y.; Ma, R. J.; Shi, L. Q.; An, Y. L. Tpps aggregates with chirality induced by hydrogen bonds from polycationic glycoconjugate. Acta Polymerica Sinica (in Chinese) 2014, 1378−1385.
Phenylboronic acid based glucose-responsive polymeric materials for insulin delivery and glucose monitoring
Acta Polymerica Sinica (in Chinese) 2014 1161 1173Liu, G.; Yang, H.; Ma, R. J.; Shi, L. Q. Phenylboronic acid based glucose-responsive polymeric materials for insulin delivery and glucose monitoring. Acta Polymerica Sinica (in Chinese) 2014, 1161−1173.
Protein refolding assisted by thermosensitive complex micelles
Acta Polymerica Sinica (in Chinese) 2014 1561 1567Zhang, H. X.; Song, Y. Q.; An, Y. L.; Wang, Y.; Shi, L. Q. Protein refolding assisted by thermosensitive complex micelles. Acta Polymerica Sinica (in Chinese) 2014, 1561−1567.
, Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms
ACS Nano 2016 10 4779 4789Liu, Y.; Busscher, H. J.; Zhao, B.; Li, Y.; Zhang, Z.; van der Mei, H. C.; Ren, Y.; Shi, L. , Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano 2016, 10, 4779−4789.
Silver-decorated polymeric micelles combined with curcumin for enhanced antibacterial activity
ACS Appl. Mater. Interfaces 2017 9 16880 16889Huang, F.; Gao, Y.; Zhang, Y.; Cheng, T.; Ou, H.; Yang, L.; Liu, J.; Shi, L.; Liu, J. Silver-decorated polymeric micelles combined with curcumin for enhanced antibacterial activity. ACS Appl. Mater. Interfaces 2017, 9, 16880−16889.
Versatile pH-responsive chitosan-g-polycaprolactone/maleic anhydride-isoniazid polymeric micelle to improve the bioavailability of tuberculosis multidrugs
ACS Appl. Bio Mater. 2019 2 1931 1943Praphakar, R. A.; Ebenezer, R. S.; Vignesh, S.; Shakila, H.; Rajan, M. Versatile pH-responsive chitosan-g-polycaprolactone/maleic anhydride-isoniazid polymeric micelle to improve the bioavailability of tuberculosis multidrugs. ACS Appl. Bio Mater. 2019, 2, 1931−1943.
Polypeptidic micelles stabilized with sodium alginate enhance the activity of encapsulated bedaquiline
Macromol. Biosci. 2019 19 1800397Soria-Carrera, H.; Lucia, A.; De Matteis, L.; Ainsa, J. A.; de la Fuente, J. M.; Martin-Rapun, R. Polypeptidic micelles stabilized with sodium alginate enhance the activity of encapsulated bedaquiline. Macromol. Biosci. 2019, 19, 1800397.
Poly(β-amino esters): synthesis, formulations, and their biomedical applications
Adv. Healthc. Mater. 2019 8 1801359Liu, Y.; Li, Y.; Keskin, D.; Shi, L. Poly(β-amino esters): synthesis, formulations, and their biomedical applications. Adv. Healthc. Mater. 2019, 8, 1801359.
Eradication of multidrug-resistant staphylococcal infections by light-activatable micellar nanocarriers in a murine model
Adv. Funct. Mater. 2017 27 1701974Liu, Y.; van der Mei, H. C.; Zhao, B.; Zhai, Y.; Cheng, T.; Li, Y.; Zhang, Z.; Busscher, H. J.; Ren, Y.; Shi, L. Eradication of multidrug-resistant staphylococcal infections by light-activatable micellar nanocarriers in a murine model. Adv. Funct. Mater. 2017, 27, 1701974.
Design of polymeric nanoparticles for biomedical delivery applications
Chem. Soc. Rev. 2012 41 2545 2561Elsabahy, M.; Wooley, K. L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545−2561.
Nanotechnology as a therapeutic tool to combat microbial resistance
Adv. Drug Del. Rev. 2013 65 1803 1815Pelgrift, R. Y.; Friedman, A. J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Del. Rev. 2013, 65, 1803−1815.
Nanocarriers with conjugated antimicrobials to eradicate pathogenic biofilms evaluated in murine in vivo and human ex vivo infection models
Acta Biomater. 2018 79 331 343Liu, Y.; Ren, Y.; Li, Y.; Su, L.; Zhang, Y.; Huang, F.; Liu, J.; Liu, J.; van Kooten, T. G.; An, Y.; Shi, L.; van der Mei, H. C.; Busscher, H. J. Nanocarriers with conjugated antimicrobials to eradicate pathogenic biofilms evaluated in murine in vivo and human ex vivo infection models. Acta Biomater. 2018, 79, 331−343.
Designing nanogel carriers for antibacterial applications
Acta Biomater. 2014 10 2105 2111Ferrer, M. C. C.; Dastghey, S.; Hickok, N. J.; Eckrnann, D. M.; Composto, R. J. Designing nanogel carriers for antibacterial applications. Acta Biomater. 2014, 10, 2105−2111.
The use of poly(methacrylic acid) nanogel to control the release of amoxycillin with lower cytotoxicity
Mater. Sci. Eng. C-Mater. Biol. Appl. 2014 43 622 629Liu, T.; Liu, H. X.; Wu, Z. M.; Chen, T.; Zhou, L.; Liang, Y. Y.; Me, B.; Huang, H. X.; Jiang, Z. Y.; Xie, M. Q.; Wu, T. The use of poly(methacrylic acid) nanogel to control the release of amoxycillin with lower cytotoxicity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 43, 622−629.
Design and in vitro evaluation of a novel poly(methacrylic acid)/metronidazole antibacterial nanogel as an oral dosage form
Colloid. Surf. B 2014 118 65 71Chen, T.; Chen, L.; Li, H. C.; Chen, Y. H.; Guo, H. X.; Shu, Y.; Chen, Z. Y.; Cai, C. H.; Guo, L. N.; Zhang, X. N.; Zhou, L.; Zhong, Q. Design and in vitro evaluation of a novel poly(methacrylic acid)/metronidazole antibacterial nanogel as an oral dosage form. Colloid. Surf. B 2014, 118, 65−71.
Hydrogels in biology and medicine: from molecular principles to bionanotechnology
Adv. Mater. 2006 18 1345 1360Peppas, N. A.; Hilt, J. Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345−1360.
The development of microgels/nanogels for drug delivery applications
Prog. Polym. Sci. 2008 33 448 477Oh, J. K.; Drumright, R.; Siegwart, D. J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448−477.
Stimuli-responsive nanogel composites and their application in nanomedicine
Chem. Soc. Rev. 2015 44 6161 6186Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderon, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161−6186.
Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications
Macromol. Rapid Commun. 2018 39 1800069Hu, Y.; Zhang, Z. Y.; Li, Y.; Ding, X. K.; Li, D. W.; Shen, C. N.; Xu, F. J. Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol. Rapid Commun. 2018, 39, 1800069.
Hemostatic porous sponges of cross-linked hyaluronic acid/cationized dextran by one self-foaming process
Mater. Sci. Eng. C-Mater. Biol. Appl. 2018 83 160 168Liu, J. Y.; Li, Y.; Hu, Y.; Cheng, G.; Ye, E. Y.; Shen, C. A.; Xu, F. J. Hemostatic porous sponges of cross-linked hyaluronic acid/cationized dextran by one self-foaming process. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 83, 160−168.
Core-shell supramolecular gelatin nanoparticles for adaptive and "on-demand" antibiotic delivery
ACS Nano 2014 8 4975 4983Li, L. L.; Xu, J. H.; Qi, G. B.; Zhao, X. Z.; Yu, F. Q.; Wang, H. Core-shell supramolecular gelatin nanoparticles for adaptive and "on-demand" antibiotic delivery. ACS Nano 2014, 8, 4975−4983.
Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections
Front. Chem. 2020 7 872Wang, D. Y.; van der Mei, H. C.; Ren, Y.; Busscher, H. J.; Shi, L. Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front. Chem. 2020, 7, 872.
Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa
Antimicrob. Agents Chemother. 2006 50 2016 2022Mugabe, C.; Halwani, M.; Azghani, A. O.; Lafrenie, R. M.; Omri, A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2016−2022.
Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients
J. Antimicrob. Chemother. 2005 55 269 271Mugabe, C.; Azghani, A. O.; Omri, A. Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J. Antimicrob. Chemother. 2005, 55, 269−271.
Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells
Biochim. Biophys. Acta-Biomembr. 2000 1463 254 266Sachetelli, S.; Khalil, H.; Chen, T.; Beaulac, C.; Senechal, S.; Lagace, J. Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim. Biophys. Acta-Biomembr. 2000, 1463, 254−266.
Pharmacokinetics and efficacies of liposomal and conventional formulations of tobramycin after intratracheal administration in rats with pulmonary Burkholdefia cepacia infection
Antimicrob. Agents Chemother. 2002 46 3776 3781Marier, J. F.; Lavigne, J.; Ducharme, M. P. Pharmacokinetics and efficacies of liposomal and conventional formulations of tobramycin after intratracheal administration in rats with pulmonary Burkholdefia cepacia infection. Antimicrob. Agents Chemother. 2002, 46, 3776−3781.
Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms
PLoS One 2013 8 e79220Messiaen, A. S.; Forier, K.; Nelis, H.; Braeckmans, K.; Coenye, T. Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS One 2013, 8, e79220.
Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria
Int. J. Antimicrob. Agents 2010 35 553 558Nicolosi, D.; Scalia, M.; Nicolosi, V. M.; Pignatello, R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553−558.
In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus
Int. J. Pharm. 2012 436 659 676Chakraborty, S. P.; Sahu, S. K.; Pramanik, P.; Roy, S. In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus. Int. J. Pharm. 2012, 436, 659−676.
Antimicrobial properties of liposomal azithromycin for Pseudomonas infections in cystic fibrosis patients
J. Antimicrob. Chemother. 2015 70 784 796Solleti, V. S.; Alhariri, M.; Halwani, M.; Omri, A. Antimicrobial properties of liposomal azithromycin for Pseudomonas infections in cystic fibrosis patients. J. Antimicrob. Chemother. 2015, 70, 784−796.
Preparation, characterization and in vitro antimicrobial activity of metronidazole bearing lectinized liposomes for intra-periodontal pocket delivery
Pharmazie 2001 56 554 560Vyas, S. P.; Sihorkar, V.; Dubey, P. K. Preparation, characterization and in vitro antimicrobial activity of metronidazole bearing lectinized liposomes for intra-periodontal pocket delivery. Pharmazie 2001, 56, 554−560.
Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections
J. Antimicrob. Chemother. 2008 61 859 868Meers, P.; Neville, M.; Malinin, V.; Scotto, A. W.; Sardaryan, G.; Kurumunda, R.; Mackinson, C.; James, G.; Fisher, S.; Perkins, W. R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 2008, 61, 859−868.
A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment
Int. J. Biol. Macromol. 2019 129 1113 1119Hu, F.; Zhou, Z.; Xu, Q.; Fan, C.; Wang, L.; Ren, H.; Xu, S.; Ji, Q.; Chen, X. A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int. J. Biol. Macromol. 2019, 129, 1113−1119.
pH-Activated nanoparticles with targeting for the treatment of oral plaque biofilm
J. Mater. Chem. B 2018 6 586 592Zhou, Z.; Hu, F.; Hu, S.; Kong, M.; Feng, C.; Liu, Y.; Cheng, X.; Ji, Q.; Chen, X. pH-Activated nanoparticles with targeting for the treatment of oral plaque biofilm. J. Mater. Chem. B 2018, 6, 586−592.
Antimicrobial synergy of monolaurin lipid nanocapsules with adsorbed antimicrobial peptides against Staphylococcus aureus biofilms in vitro is absent in vivo
J. Control. Release 2019 293 73 83Rozenbaum, R. T.; Su, L.; Umerska, A.; Eveillard, M.; Hakansson, J.; Mahlapuu, M.; Huang, F.; Liu, J.; Zhang, Z.; Shi, L.; van der Mei, H. C.; Busscher, H. J.; Sharma, P. K. Antimicrobial synergy of monolaurin lipid nanocapsules with adsorbed antimicrobial peptides against Staphylococcus aureus biofilms in vitro is absent in vivo. J. Control. Release 2019, 293, 73−83.
Liposome: classification, preparation, and applications
Nanoscale Res. Lett 2013 8 102Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S. W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett 2013, 8, 102.
Design principles, synthesis and biomedical applications of polymer vesicles with inhomogeneous membranes
J. Control. Release 2020 326 365 386Liu, D. Q.; Sun, H.; Xiao, Y. F.; Chen, S.; Cornel, E. J.; Zhu, Y. Q.; Du, J. Z. Design principles, synthesis and biomedical applications of polymer vesicles with inhomogeneous membranes. J. Control. Release 2020, 326, 365−386.
Dual corona vesicles with intrinsic antibacterial and enhanced antibiotic delivery capabilities for effective treatment of biofilm-induced periodontitis
ACS Nano 2019 13 13645 13657Xi, Y.; Wang, Y.; Gao, J.; Xiao, Y.; Du, J. Dual corona vesicles with intrinsic antibacterial and enhanced antibiotic delivery capabilities for effective treatment of biofilm-induced periodontitis. ACS Nano 2019, 13, 13645−13657.
Size and charge adaptive clustered nanoparticles targeting the biofilm microenvironment for chronic lung infection management
ACS Nano 2020 14 5686 5699Gao, Y.; Wang, J.; Chai, M.; Li, X.; Deng, Y.; Jin, Q.; Ji, J. Size and charge adaptive clustered nanoparticles targeting the biofilm microenvironment for chronic lung infection management. ACS Nano 2020, 14, 5686−5699.
Antimicrobial peptide therapeutics for cystic fibrosis
Antimicrob. Agents Chemother. 2005 49 2921 2927Zhang, L. J.; Parente, J.; Harris, S. A.; Woods, D. E.; Hancock, R. E. W.; Fallal, T. J. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 2005, 49, 2921−2927.
Antifungal activity from 14-helical β-peptides
J. Am. Chem. Soc. 2006 128 12630 12631Karlsson, A. J.; Pomerantz, W. C.; Weisblum, B.; Gellman, S. H.; Palecek, S. P. Antifungal activity from 14-helical β-peptides. J. Am. Chem. Soc. 2006, 128, 12630−12631.
Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies
Nat. Biotechnol. 2006 24 1551 1557Hancock, R. E. W.; Sahl, H. G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551−1557.
Cationic peptides: effectors in innate immunity and novel antimicrobials
Lancet Infect. Dis. 2001 1 156 164Hancock, R. E. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156−164.
Diversity of antimicrobial peptides and their mechanisms of action
Biochim. Biophys. Acta-Biomembr. 1999 1462 11 28Epand, R. M.; Vogel, H. J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta-Biomembr. 1999, 1462, 11−28.
Living polypeptides
Biomacromolecules 2004 5 1653 1656Aliferis, T.; Iatrou, H.; Hadjichristidis, N. Living polypeptides. Biomacromolecules 2004, 5, 1653−1656.
Antimicrobial peptides of multicellular organisms
Nature 2002 415 389 395Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389−395.
Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers
Nat. Microbiol 2016 1 16162Lam, S. J.; O'Brien-Simpson, N. M.; Pantarat, N.; Sulistio, A.; Wong, E. H.; Chen, Y. Y.; Lenzo, J. C.; Holden, J. A.; Blencowe, A.; Reynolds, E. C.; Qiao, G. G. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol 2016, 1, 16162.
Antibacterial polypeptide-grafted chitosan-based nanocapsules as an “armed” carrier of anticancer and antiepileptic drugs
ACS Macro Lett. 2013 2 1021 1025Zhou, C.; Wang, M.; Zou, K.; Chen, J.; Zhu, Y.; Du, J. Antibacterial polypeptide-grafted chitosan-based nanocapsules as an “armed” carrier of anticancer and antiepileptic drugs. ACS Macro Lett. 2013, 2, 1021−1025.
Biomimetic antimicrobial polymers: recent advances in molecular design
Polym. Chem. 2018 9 2407 2427Ergene, C.; Yasuhara, K.; Palermo, E. F. Biomimetic antimicrobial polymers: recent advances in molecular design. Polym. Chem. 2018, 9, 2407−2427.
Macromolecular-clustered facial amphiphilic antimicrobials
Nat. Commun. 2018 9 5231Rahman, M. A.; Bam, M.; Luat, E.; Jui, M. S.; Ganewatta, M. S.; Shokfai, T.; Nagarkatti, M.; Decho, A. W.; Tang, C. Macromolecular-clustered facial amphiphilic antimicrobials. Nat. Commun. 2018, 9, 5231.
Bacteria-assisted activation of antimicrobial polypeptides by a random-coil to helix transition
Angew. Chem. Int. Ed. 2017 56 10826 10829Xiong, M.; Han, Z.; Song, Z.; Yu, J.; Ying, H.; Yin, L.; Cheng, J. Bacteria-assisted activation of antimicrobial polypeptides by a random-coil to helix transition. Angew. Chem. Int. Ed. 2017, 56, 10826−10829.
Selective killing of Helicobacter pylori with pH-responsive helix-coil conformation transitionable antimicrobial polypeptides
Proc. Natl. Acad. Sci. U. S. A. 2017 114 12675 12680Xiong, M. H.; Bao, Y.; Xu, X.; Wang, H.; Han, Z. Y.; Wang, Z. Y.; Liu, Y. Q.; Huang, S. Y.; Song, Z. Y.; Chen, J. J.; Peek, R. M.; Yin, L. C.; Chen, L. F.; Cheng, J. J. Selective killing of Helicobacter pylori with pH-responsive helix-coil conformation transitionable antimicrobial polypeptides. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 12675−12680.
Biofilm dispersion
Biofilm Highlights 2011 5 571Davies, D. G. Biofilm dispersion. Biofilm Highlights 2011, 5, 571.
Approaches to dispersing medical biofilms
Microorganisms 2017 5 15Fleming, D.; Rumbaugh, K. P. Approaches to dispersing medical biofilms. Microorganisms 2017, 5, 15.
Biofilm dispersal: multiple elaborate strategies for dissemination of bacteria with unique properties
Mol. Microbiol. 2017 105 188 210Guilhen, C.; Forestier, C.; Balestrino, D. Biofilm dispersal: multiple elaborate strategies for dissemination of bacteria with unique properties. Mol. Microbiol. 2017, 105, 188−210.
Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci
J. Antibiot. 2012 65 73 77Kaplan, J. B.; LoVetri, K.; Cardona, S. T.; Madhyastha, S.; Sadovskaya, I.; Jabbouri, S.; Izano, E. A. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J. Antibiot. 2012, 65, 73−77.
Staphylococcus aureus biofilms: recent developments in biofilm dispersal
Front. Cell. Infect. Microbiol. 2014 4 178Lister, J. L.; Horswill, A. R. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178.
A Functional DNase I coating to prevent adhesion of bacteria and the formation of biofilm
Adv. Funct. Mater. 2013 23 2843 2849Swartjes, J. J. T. M.; Das, T.; Sharifi, S.; Subbiahdoss, G.; Sharma, P. K.; Krom, B. P.; Busscher, H. J.; van der Mei, H. C. A Functional DNase I coating to prevent adhesion of bacteria and the formation of biofilm. Adv. Funct. Mater. 2013, 23, 2843−2849.
Low temperature synthesis of superparamagnetic iron oxide (Fe3O4) nanoparticles and their ROS mediated inhibition of biofilm formed by food-associated bacteria
Front. Microbiol. 2018 9 2567Al-Shabib, N. A.; Husain, F. M.; Ahmed, F.; Khan, R. A.; Khan, M. S.; Ansari, F. A.; Alam, M. Z.; Ahmed, M. A.; Khan, M. S.; Baig, M. H.; Khan, J. M.; Shahzad, S. A.; Arshad, M.; Alyousef, A.; Ahmad, I. Low temperature synthesis of superparamagnetic iron oxide (Fe3O4) nanoparticles and their ROS mediated inhibition of biofilm formed by food-associated bacteria. Front. Microbiol. 2018, 9, 2567.
Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination
Nanoscale 2014 6 2588 2593Gao, L.; Giglio, K. M.; Nelson, J. L.; Sondermann, H.; Travis, A. J. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale 2014, 6, 2588−2593.
Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo
Biomaterials 2016 101 272 284Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P. C.; Cormode, D. P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272−284.
A H2O2-free depot for treating bacterial infection: localized cascade reactions to eradicate biofilms in vivo
Nanoscale 2018 10 17656 17662Yan, Z.; Bing, W.; Ding, C.; Dong, K.; Ren, J.; Qu, X. A H2O2-free depot for treating bacterial infection: localized cascade reactions to eradicate biofilms in vivo. Nanoscale 2018, 10, 17656−17662.
Efficient phosphodiester cleaving nanozymes resulting from multivalency and local medium polarity control
J. Am. Chem. Soc. 2014 136 1158 1161Diez-Castellnou, M.; Mancin, F.; Scrimin, P. Efficient phosphodiester cleaving nanozymes resulting from multivalency and local medium polarity control. J. Am. Chem. Soc. 2014, 136, 1158−1161.
Designed α-sheet peptides suppress amyloid formation in Staphylococcus aureus biofilms
Npj Biofilms and Microbiomes. 2017 3 16Bleem, A.; Francisco, R.; Bryers, J. D.; Daggett, V. Designed α-sheet peptides suppress amyloid formation in Staphylococcus aureus biofilms. Npj Biofilms and Microbiomes. 2017, 3, 16.
Broad-spectrum anti-biofilm peptide that targets a cellular stress response
PLoS Path. 2014 10 e1004152De la Fuente-Nunez, C.; Reffuveille, F.; Haney, E. F.; Straus, S. K.; Hancock, R. E. W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Path. 2014, 10, e1004152.
De Novo designed α-sheet peptides inhibit functional amyloid formation of Streptococcus mutans biofilms
J. Mol. Biol. 2018 430 3764 3773Paranjapye, N.; Daggett, V. De Novo designed α-sheet peptides inhibit functional amyloid formation of Streptococcus mutans biofilms. J. Mol. Biol. 2018, 430, 3764−3773.
Biofilm disruption utilizing α/β chimeric polypeptide molecular brushes
Chinese J. Polym. Sci. 2019 37 1105 1112Zhang, S.; Xiao, X. M.; Qi, F.; Ma, P. C.; Zhang, W. W.; Dai, C. Z.; Zhang, D. F.; Liu, R. H. Biofilm disruption utilizing α/β chimeric polypeptide molecular brushes. Chinese J. Polym. Sci. 2019, 37, 1105−1112.
2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms
Angew. Chem. Int. Ed. 2012 51 5226 5229Frei, R.; Breitbach, A. S.; Blackwell, H. E. 2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms. Angew. Chem. Int. Ed. 2012, 51, 5226−5229.
Bacterial quorum-sensing network architectures
Annu. Rev. Genet. 2009 43 197 222Ng, W.-L.; Bassler, B. L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197−222.
D-amino acids trigger biofilm disassembly
Science 2010 328 627 629Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-amino acids trigger biofilm disassembly. Science 2010, 328, 627−629.
Lipopeptide biosurfactant viscosin enhances dispersal of Pseudomonas fluorescens SBW25 biofilms
Microbiology-Sgm. 2015 161 2289 2297Bonnichsen, L.; Svenningsen, N. B.; Rybtke, M.; de Bruijn, I.; Raaijmakers, J. M.; Tolker-Nielsen, T.; Nybroe, O. Lipopeptide biosurfactant viscosin enhances dispersal of Pseudomonas fluorescens SBW25 biofilms. Microbiology-Sgm. 2015, 161, 2289−2297.
Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1
J. Bacteriol. 2003 185 1027 1036Davey, M. E.; Caiazza, N. C.; O'Toole, G. A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003, 185, 1027−1036.
Antimicrobial effects of an NO-releasing poly(ethylene vinylacetate) coating on soft-tissue implants in vitro and in a murine model
Acta Biomater. 2009 5 1905 1910Engelsman, A. F.; Krom, B. P.; Busscher, H. J.; van Dam, G. M.; Ploeg, R. J.; van der Mei, H. C. Antimicrobial effects of an NO-releasing poly(ethylene vinylacetate) coating on soft-tissue implants in vitro and in a murine model. Acta Biomater. 2009, 5, 1905−1910.
Bacterial biofilm destruction by size/surface charge-adaptive micelles
Nanoscale 2019 11 1410 1422Chen, M.; Wei, J.; Xie, S.; Tao, X.; Zhang, Z.; Ran, P.; Li, X. Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale 2019, 11, 1410−1422.
Encapsulated DNase improving the killing efficiency of antibiotics in staphylococcal biofilms
J. Mater. Chem. B 2020 8 4395 4401Liu, C.; Zhao, Y.; Su, W.; Chai, J.; Xu, L.; Cao, J.; Liu, Y. Encapsulated DNase improving the killing efficiency of antibiotics in staphylococcal biofilms. J. Mater. Chem. B 2020, 8, 4395−4401.
Magnetic glycol chitin-based hydrogel nanocomposite for combined thermal and D-amino-acid-assisted biofilrn disruption
ACS Infect. Dis. 2018 4 1246 1256Abenojar, E. C.; Wickramasinghe, S.; Ju, M.; Uppaluri, S.; Klika, A.; George, J.; Barsoum, W.; Frangiamore, S. J.; Higuera-Rueda, C. A.; Samia, A. C. S. Magnetic glycol chitin-based hydrogel nanocomposite for combined thermal and D-amino-acid-assisted biofilrn disruption. ACS Infect. Dis. 2018, 4, 1246−1256.
Functional gold nanoparticles for the storage and controlled release of nitric oxide: applications in biofilm dispersal and intracellular delivery
J. Mater. Chem. B 2014 2 5003 5011Duong, H. T. T.; Adnan, N. N. M.; Barraud, N.; Basuki, J. S.; Kutty, S. K.; Jung, K.; Kumar, N.; Davis, T. P.; Boyer, C. Functional gold nanoparticles for the storage and controlled release of nitric oxide: applications in biofilm dispersal and intracellular delivery. J. Mater. Chem. B 2014, 2, 5003−5011.
PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix
Mater. Sci. Eng. C-Mater. Biol. Appl. 2019 103 109741Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S. P.; Kim, J.; Jung, Y.; Lee, B. L.; Yoo, J. W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 103, 109741.
Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles
Chem. Sci. 2016 7 1016 1027Nguyen, T. K.; Selvanayagam, R.; Ho, K. K. K.; Chen, R.; Kutty, S. K.; Rice, S. A.; Kumar, N.; Barraud, N.; Duong, H. T. T.; Boyer, C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016, 7, 1016−1027.
Enhancing the stability and antibiofilm activity of DspB by immobilization on carboxymethyl chitosan nanoparticles
Microbiol. Res. 2015 178 35 41Tan, Y.; Ma, S.; Liu, C.; Yu, W.; Han, F. Enhancing the stability and antibiofilm activity of DspB by immobilization on carboxymethyl chitosan nanoparticles. Microbiol. Res. 2015, 178, 35−41.
DNase-I functionalization of ciprofloxacin-loaded chitosan nanoparticles overcomes the biofilm-mediated resistance of Pseudomonas aeruginosa
Appl. Nanosci. 2020 10 563 575Patel, K. K.; Agrawal, A. K.; Anjum, M. M.; Tripathi, M.; Pandey, N.; Bhattacharya, S.; Tilak, R.; Singh, S. DNase-I functionalization of ciprofloxacin-loaded chitosan nanoparticles overcomes the biofilm-mediated resistance of Pseudomonas aeruginosa. Appl. Nanosci. 2020, 10, 563−575.
Aggregation and interaction of cationic nanoparticles on bacterial surfaces
J. Am. Chem. Soc. 2012 134 6920 3Hayden, S. C.; Zhao, G.; Saha, K.; Phillips, R. L.; Li, X.; Miranda, O. R.; Rotello, V. M.; El-Sayed, M. A.; Schmidt-Krey, I.; Bunz, U. H. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J. Am. Chem. Soc. 2012, 134, 6920−3.
Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking
Small 2013 9 1521 1532Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521−1532.
Self-targeting, zwitterionic micellar dispersants enhance antibiotic killing of infectious biofilms—an intravital imaging study in mice
Sci. Adv. 2020 6 eabb1112Tian, S.; Su, L.; Liu, Y.; Cao, J.; Yang, G.; Ren, Y.; Huang, F.; Liu, J.; An, Y.; van der Mei, H. C.; Busscher, H. J.; Shi, L. Self-targeting, zwitterionic micellar dispersants enhance antibiotic killing of infectious biofilms—an intravital imaging study in mice. Sci. Adv. 2020, 6, eabb1112.
Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms
ACS Nano. 2020 14 347 359Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms. ACS Nano. 2020, 14, 347−359.
Cargo-templated crosslinked polymer nanocapsules and their biomedical applications
Adv. NanoBiomed Res. 2021 1 2000078Zhao, Y.; Li, Q.; Chai, J.; Liu, Y. Cargo-templated crosslinked polymer nanocapsules and their biomedical applications. Adv. NanoBiomed Res. 2021, 1, 2000078.
Coating of a novel antimicrobial nanoparticle with a macrophage membrane for the selective entry into infected macrophages and killing of intracellular Staphylococci
Adv. Funct. Mater. 2020 30 2004942Li, Y.; Liu, Y.; Ren, Y.; Su, L.; Li, A.; An, Y.; Rotello, V.; Zhang, Z.; Wang, Y.; Liu, Y.; Liu, S.; Liu, J.; Laman, J. D.; Shi, L.; Mei, H. C.; Busscher, H. J. Coating of a novel antimicrobial nanoparticle with a macrophage membrane for the selective entry into infected macrophages and killing of intracellular Staphylococci. Adv. Funct. Mater. 2020, 30, 2004942.
Applications and perspectives of cascade reactions in bacterial infection control
Front. Chem. 2020 7 861Li, Y.; Yang, G.; Ren, Y.; Shi, L.; Ma, R.; van der Mei, H. C.; Busscher, H. J. Applications and perspectives of cascade reactions in bacterial infection control. Front. Chem. 2020, 7, 861.