Ion specificities are ubiquitous in various systems including biology,[1−3] colloids,[4−6] and polymers.[7−14] Since the Czech scientist Franz Hofmeister firstly discovered these ion-specific effects more than 100 years ago, the ion specificities have been extensively investigated by the experimentalists and theoreticians in a wide range of fields.[15−25] In general, the ion specificities of polymers in aqueous solutions can be observed only at high salt concentrations (typically above 0.1 mol/L), where the long-range nonspecific electrostatic interactions are effectively screened due to the small Debye length, and the properties of the polymer systems are dominated by the short-range ion-specific interactions.[13,26,27] However, a few previous studies have demonstrated that the specific ion effects on polymers can also be observed at low salt concentrations (typically around millimolar salt concentrations).[28−30] The reason why a similar strength of ion specificities can be observed at very different salt concentrations for different polymer systems remains elusive.
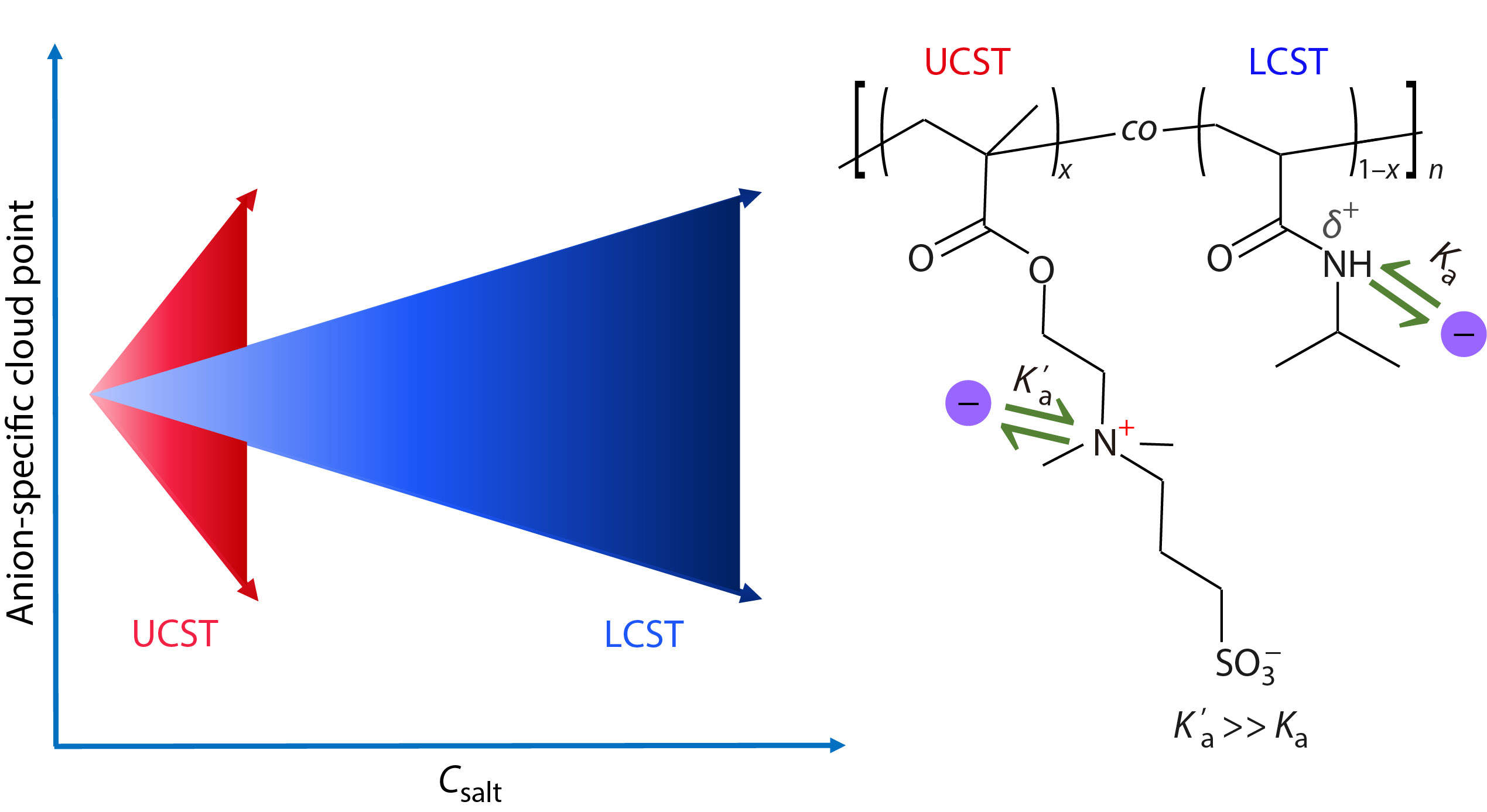
For instance, the obvious specific ion effects on neutral polymers are usually observed at the salt concentrations higher than ~100 mmol/L, whereas the ion specificities of charged polymers can be obviously observed at the salt concentrations as low as ~0.1−1 mmol/L.[13,26,28−33] We hypothesize that the strength of ion specificities of polymers is closely related to the affinity of direct ion binding to the polymers. Specifically, a higher ion binding affinity leads more ions to bind to the polymers at a certain ion concentration, such that the different types of ions can be more effectively distinguished and a stronger ion specificity of the polymers is resulted. It is well-known that the neutral poly(N-isopropylacrylamide) (PNIPAM) and the charged poly(sulfobetaine methacrylate) (PSBMA) exhibit the lower critical solution temperature (LCST) and the upper critical solution temperature (UCST) behaviors in aqueous solutions, respectively (Fig. S1 in the electronic supplementary information, ESI).[26,34−37] To test the above hypothesis, we have prepared a series of statistical copolymers of poly(sulfobetaine methacrylate-co-N-isopropylacrylamide) (P(SBMA-co-NIPAM)) with either the UCST or the LCST behavior (Fig. 1 and Table S1 in ESI), where the anions exhibit a much stronger binding to the positively charged quaternary ammonium groups of the SBMA units (Ka’) than that to the partially positively charged amide groups of the NIPAM units (Ka) according to our previous study.[38] In this work, our aim is to clarify the reason why a similar strength of anion specificities can be observed at very different salt concentrations for different types of thermosensitive polymers.
Fig. 2(a) shows the salt concentration (Csalt) dependence of cloud point temperature (Tcp) of the PNIPAM aqueous solutions in the presence of different types of anions with the Na+ as the common cation. The specific anion effects on the thermoresponsive behavior of PNIPAM observed in Fig. 2(a) are similar to those reported in the previous studies.[7,26] For example, Tcp decreases with increasing salt concentrations for Cl− and NO3−, whereas it initially increases and then decreases with increasing salt concentration for SCN−. The specific anion effects on Tcp of PNIPAM can be well understood on the basis of the model proposed by Cremer et al. consisting of three kinds of interactions between anions, PNIPAM, and water molecules.[26] The specific anion effects on Tcp of P(SBMA0.11-co-NIPAM0.89) and P(SBMA0.19-co-NIPAM0.81) are shown in Figs. 2(b) and 2(c). It is evident that the specific anion effects on Tcp are amplified with increasing salt concentration from 0 mol/L to 1.0 mol/L for all the LCST-type polymers. As the molar fraction of SBMA (x) increases, the value of Tcp increases for the same type of anion at the same salt concentration due to the enhanced polymer hydrophilicity by incorporating more SBMA units into the polymers. For the same type of anion, the salt concentration dependence of salting-in and salting-out effects are respectively strengthened and weakened with increasing x for the LCST-type polymers. This is because the solubility of PSBMA is enhanced with increasing salt concentration[39] and a higher x would give rise to a more obvious increase in solubility of the LCST-type polymers with increasing salt concentration.
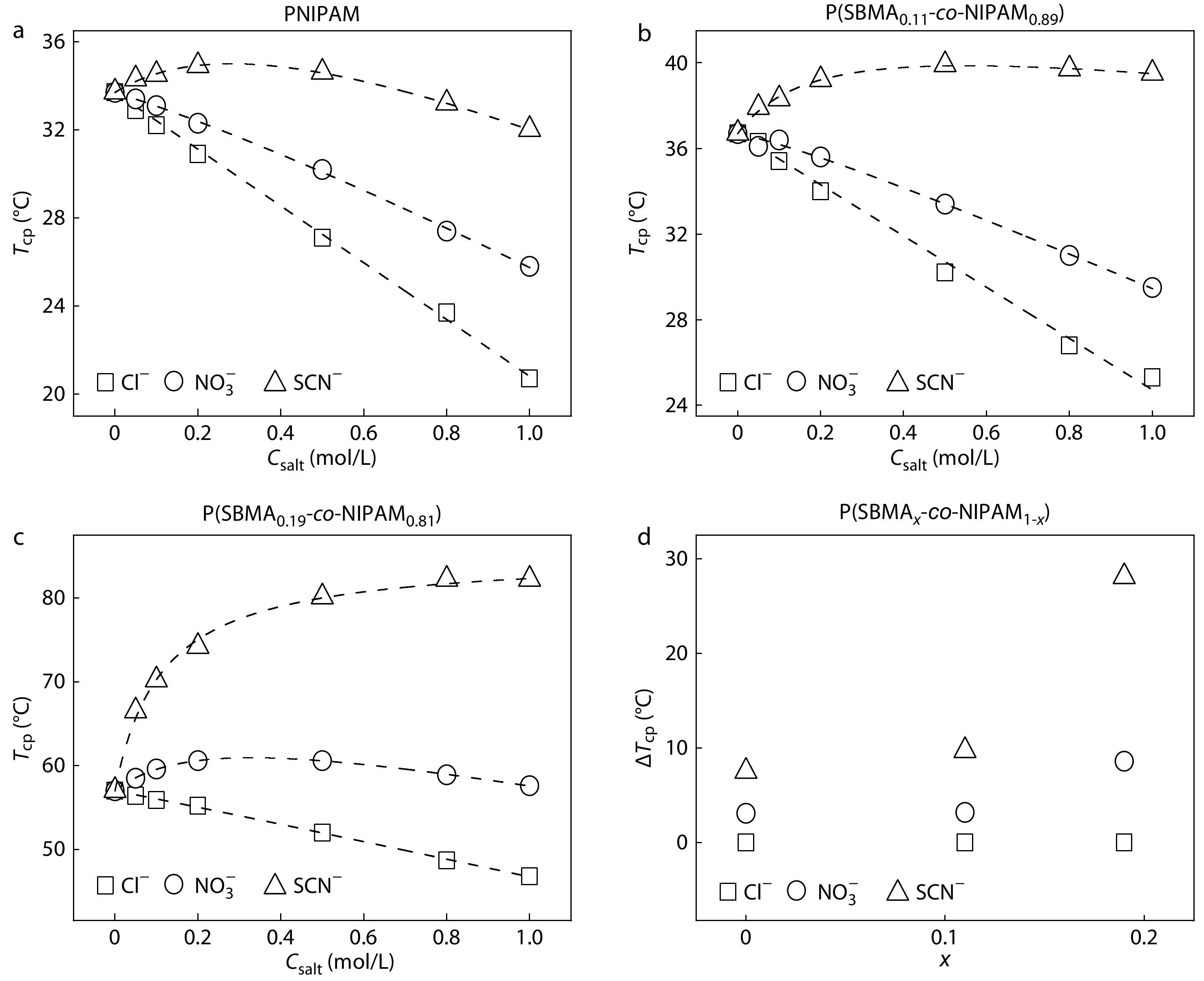
More importantly, the specific anion effects on Tcp of the LCST-type polymers can be amplified by increasing x, as reflected by the difference in Tcp (ΔTcp) between the anions, which is amplified with an increase in x at 0.5 mol/L (Fig. 2d). Similar amplifications of ΔTcp between the anions for the LCST-type polymers with increasing x are also observed at other salt concentrations (Fig. S2 in ESI). What concerns us is why the strength of anion specificities of the LCST-type polymers is enhanced with increasing x. According to the previous studies,[26,38,40,41] the direct anion-NIPAM interaction is dominated by the interaction between anions and the partially positively charged amide group, whereas the direct anion-SBMA interaction is dominated by the interaction between anions and the positively charged quaternary ammonium group. Therefore, it is reasonable to expect that the amplification of the specific anion effects on Tcp of the LCST-type polymers with increasing x should be closely related to the difference in strength between these two types of anion-polymer interactions. We will come back to this point later.
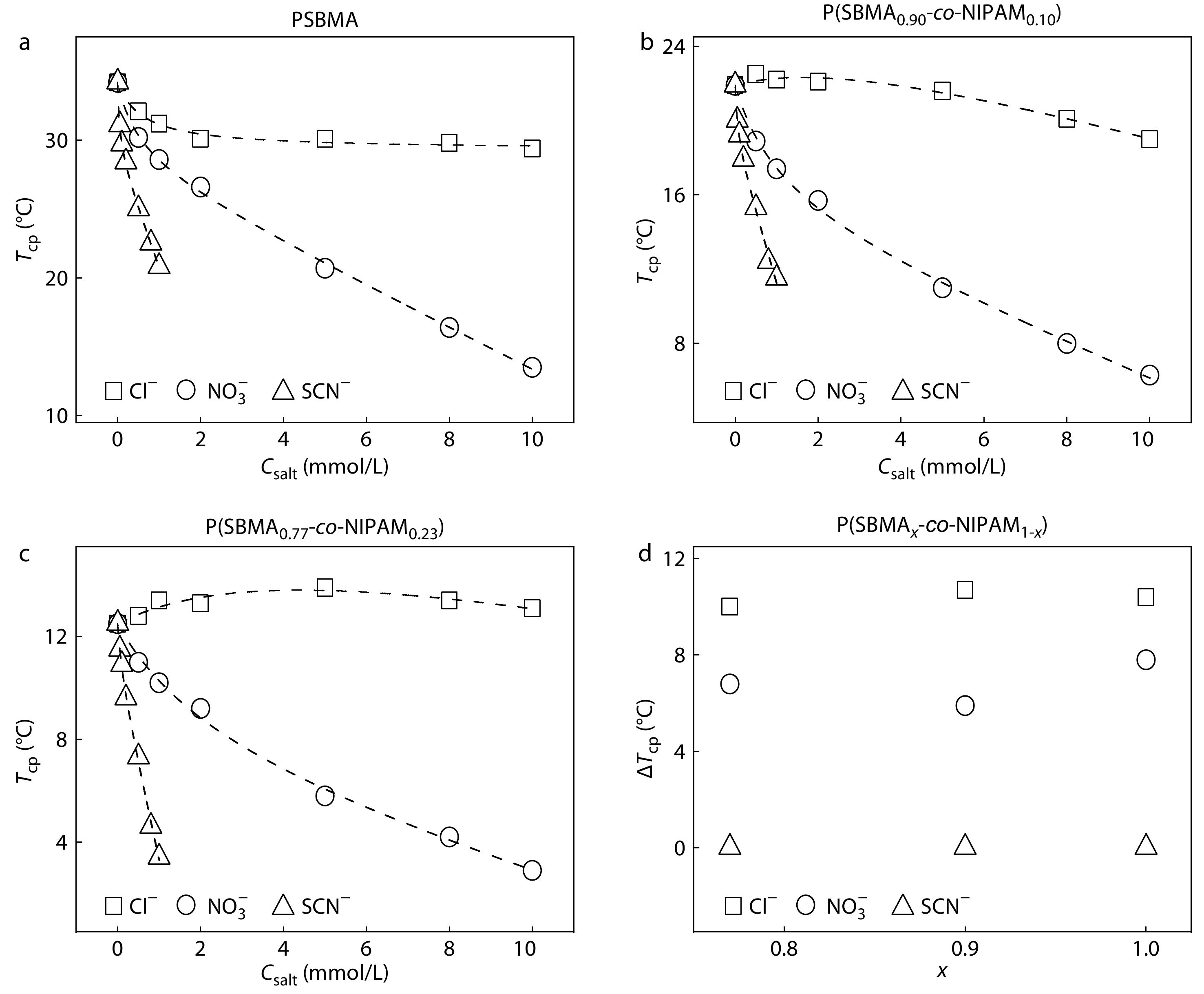
In Figs. 3(a)-3(c), the specific anion effects on Tcp of the UCST-type polymers can be observed around millimolar salt concentrations, where the anion specificities are amplified with increasing salt concentration from 0 mmol/L to 10.0 mmol/L. On the other hand, the strength of the specific anion effects on Tcp of the UCST-type polymers is only slightly influenced by x at the low salt concentrations (Fig. 3d and Fig. S3 in ESI), but is enhanced with increasing x at the relatively high salt concentrations (Fig. S3 in ESI). Again, the amplification of anion specificities of the UCST-type polymers with increasing x should be closely correlated with the difference in strength between anion-SBMA and anion-NIPAM interactions. In Fig. 2(d), the strength of specific anion effects as reflected by the ΔTcp is ~10 °C at 0.5 mol/L for PNIPAM and P(SBMA0.11-co-NIPAM0.89). A similar strength of anion specificities is observed at 1.0 mmol/L for PSBMA, P(SBMA0.90-co-NIPAM0.10), and P(SBMA0.77-co-NIPAM0.23), as shown in Fig. 3(d). That is, a 500 times higher salt concentration is required for the former two LCST-type polymers to achieve a similar strength of anion specificities with that for the latter three UCST-type polymers.
To analyze the reason why a similar strength of anion specificities can be observed at very different salt concentrations for different types of thermosensitive polymers, Eqs. (1) and (2) are respectively employed to model the salt concentration dependence of Tcp for the LCST- and UCST-type polymers (Figs. 2 and 3), where T0 is the Tcp of the polymer solutions in the absence of salt, and [M] is the molar concentration of the salts. The constant c is correlated with the salting-out effect for the LCST-type polymers and the constant c’ is related to the salting-in effect for the UCST-type polymers. Ka and Ka’ are the anion binding constants for the LCST- and UCST-type polymers, respectively. Bmax (Bmax’) represents the maximum increase (decrease) in Tcp for the LCST (UCST)-type polymers caused by the direct anion binding.[26,42] The fitting parameters are listed in Table 1, Tables S2 and S3 (in ESI).
| Anion identity | PNIPAM | P(SBMA0.11-co-NIPAM0.89) | P(SBMA0.19-co-NIPAM0.81) | |
| Ka (L/mol) | Cl− | ~0 | ~0 | 1.6 |
| NO3− | 0.9 | 2.4 | 3.9 | |
| SCN− | 1.9 | 4.7 | 8.9 | |
| Anion identity | PSBMA | P(SBMA0.90-co-NIPAM0.10) | P(SBMA0.77-co-NIPAM0.23) | |
| Ka’ (L/mol) | Cl− | 1659.2 | 235.4 | 190.3 |
| NO3− | 1967.8 | 919.5 | 395.7 | |
| SCN− | 19626.7 | 14768.2 | 7816.5 |


In Table 1, the Ka increases following the anion trend Cl−<NO3−<SCN− for the same LCST-type polymer. The direct anion binding to the LCST-type polymers is dominated by the interactions between the anions and the partially positively charged amide groups of NIPAM units. The anion specificity in terms of the values of Ka could be understood based on the theory of ionic dispersion forces, because the anionic polarizability is increased from Cl− to SCN− following the above anion trend. Furthermore, for the same kind of anion, the increasing Ka with increasing x for the LCST-type polymers indicates that a stronger anion binding is resulted as more SBMA units are incorporated into the polymers. For the UCST-type polymers, the direct anion binding to the polymers is dominated by the ion-pairing interactions between the anions and the positively charged quaternary ammonium groups of SBMA units. According to the law of matching water affinities,[43] the strength of the ion-pairing interactions as reflected by the Ka’ value should increase following the anion trend Cl−<NO3−<SCN−, as the positively charged quaternary ammonium is a weakly hydrated group and the extent of hydration of the anions decreases following the anion trend Cl−>NO3−>SCN−.[30,44] This is what exactly observed for the UCST-type polymers. Moreover, for the same type of anion, the Ka’ increases with increasing x for the UCST-type polymers, suggesting that the anion binding affinity is enhanced by incorporating more SBMA units into the polymers.
As shown in Figs. 2(d) and 3(d), the anion specificities of PNIPAM and P(SBMA0.11-co-NIPAM0.89) have a similar strength with those for PSBMA, P(SBMA0.90-co-NIPAM0.10), and P(SBMA0.77-co-NIPAM0.23), but the anion specificities of the UCST-type polymers are observed at a salt concentration 500 times lower than those of the LCST-type polymers. If we have a close look at the Table 1, we can find that the Ka’ for PSBMA is much larger than Ka for PNIPAM for the same kind of anion. As the interactions of the UCST- and LCST-type polymers with the anions are respectively dominated by the anion-SBMA and anion-NIPAM interactions, the Ka’ values for all the UCST-type polymers are much larger than those of Ka values for PNIPAM and P(SBMA0.11-co-NIPAM0.89) for the same kind of anion. Thus, in comparison with the LCST-type polymers, a similar strength of specific anion effects observed for the UCST-type polymers at a much lower salt concentration is attributed to the higher anion binding affinities for the latter polymers than the former ones.
In conclusion, a similar strength of anion specificities can be observed at very different salt concentrations for the LCST- and UCST-type polymers. The anion binding to the LCST-type polymers is dominated by the anion-NIPAM interaction, whereas the anion binding to the UCST-type polymers is dominated by the anion-SBMA interaction. As the latter interaction is much stronger than the former interaction, a similar strength of anion specificities can be observed at a much lower salt concentration for the UCST-type polymers, compared with that for the LCST-type polymers. Thus, our work demonstrates that the anion binding affinity determines the strength of specific anion effects on the thermosensitive polymers.
Hofmeister phenomena: an update on ion specificity in biology
Chem. Rev. 2012 112 2286 2322Lo Nostro, P.; Ninham, B. W. Hofmeister phenomena: an update on ion specificity in biology. Chem. Rev. 2012, 112, 2286−2322.
Hofmeister effects in biology: effect of choline addition on the salt-induced super activity of horseradish peroxidase and its implication for salt resistance of plants
J. Phys. Chem. B 2005 109 16511 16514Pinna, M. C.; Bauduin, P.; Touraud, D.; Monduzzi, M.; Ninham, B. W.; Kunz, W. Hofmeister effects in biology: effect of choline addition on the salt-induced super activity of horseradish peroxidase and its implication for salt resistance of plants. J. Phys. Chem. B 2005, 109, 16511−16514.
Hofmeister series: the hydrolytic activity of Aspergillus niger lipase depends on specific anion effects
J. Phys. Chem. B 2005 109 5406 5408Pinna, M. C.; Salis, A.; Monduzzi, M.; Ninham, B. W. Hofmeister series: the hydrolytic activity of Aspergillus niger lipase depends on specific anion effects. J. Phys. Chem. B 2005, 109, 5406−5408.
Interaction of monovalent ions with hydrophobic and hydrophilic colloids: charge inversion and ionic specificity
J. Am. Chem. Soc. 2011 133 15025 15035Calero, C.; Faraudo, J.; Bastos-González, D. Interaction of monovalent ions with hydrophobic and hydrophilic colloids: charge inversion and ionic specificity. J. Am. Chem. Soc. 2011, 133, 15025−15035.
Inversion of Hofmeister series by changing the surface of colloidal particles from hydrophobic to hydrophilic
J. Phys. Chem. C 2010 114 11133 11139Peula-García, J. M.; Ortega-Vinuesa, J. L.; Bastos-González, D. Inversion of Hofmeister series by changing the surface of colloidal particles from hydrophobic to hydrophilic. J. Phys. Chem. C 2010, 114, 11133−11139.
Ion specificity and the theory of stability of colloidal suspensions
Phys. Rev. Lett. 2011 106 167801Dos Santos, A. P.; Levin, Y. Ion specificity and the theory of stability of colloidal suspensions. Phys. Rev. Lett. 2011, 106, 167801.
Ion specificities of artificial macromolecules
Soft Matter 2017 13 68 80Liu, L. D.; Kou, R.; Liu, G. M. Ion specificities of artificial macromolecules. Soft Matter 2017, 13, 68−80.
Tuning the properties of charged polymers at the solid/liquid interface with ions
Langmuir 2019 35 3232 3247Liu, G. M. Tuning the properties of charged polymers at the solid/liquid interface with ions. Langmuir 2019, 35, 3232−3247.
Ionic effects on synthetic polymers: from solutions to brushes and gels
Soft Matter 2020 16 4087 4104Yuan, H. Y.; Liu, G. M. Ionic effects on synthetic polymers: from solutions to brushes and gels. Soft Matter 2020, 16, 4087−4104.
Microcalorimetric study of the effects of a chaotropic salt, KSCN, on the lower critical solution temperature (LCST) of aqueous poly(N-isopropylacrylamide) (PNIPA) solutions
Macromolecules 2010 43 480 487Shechter, I.; Ramon, O.; Portnaya, I.; Paz, Y.; Livney, Y. D. Microcalorimetric study of the effects of a chaotropic salt, KSCN, on the lower critical solution temperature (LCST) of aqueous poly(N-isopropylacrylamide) (PNIPA) solutions. Macromolecules 2010, 43, 480−487.
Tuning ice nucleation with counterions on polyelectrolyte brush surfaces
Sci. Adv. 2016 2 e1600345He, Z. Y.; Xie, W. J.; Liu, Z. Q.; Liu, G. M.; Wang, Z. W.; Gao, Y. Q.; Wang, J. J. Tuning ice nucleation with counterions on polyelectrolyte brush surfaces. Sci. Adv. 2016, 2, e1600345.
Interactions between macromolecules and ions: the Hofmeister series
Curr. Opin. Chem. Biol. 2006 10 658 663Zhang, Y. J.; Cremer, P. S. Interactions between macromolecules and ions: the Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658−663.
Ion-sensitive “isothermal” responsive polymers prepared in water
J. Am. Chem. Soc. 2008 130 10852 10853Magnusson, J. P.; Khan, A.; Pasparakis, G.; Saeed, A. O.; Wang, W. X.; Alexander, C. Ion-sensitive “isothermal” responsive polymers prepared in water. J. Am. Chem. Soc. 2008, 130, 10852−10853.
Thermodynamic description of Hofmeister effects on the LCST of thermosensitive polymers
J. Phys. Chem. B 2014 118 10979 10988Heyda, J.; Dzubiella, J. Thermodynamic description of Hofmeister effects on the LCST of thermosensitive polymers. J. Phys. Chem. B 2014, 118, 10979−10988.
‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts): Franz Hofmeister’s historical papers
Curr. Opin. Colloid Interface Sci. 2004 9 19 37Kunz, W.; Henle, J.; Ninham, B. W. ‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts): Franz Hofmeister’s historical papers. Curr. Opin. Colloid Interface Sci. 2004, 9, 19−37.
The present state of affairs with Hofmeister effects
Curr. Opin. Colloid Interface Sci. 2004 9 1 18Kunz, W.; Lo Nostro, P.; Ninham, B. W. The present state of affairs with Hofmeister effects. Curr. Opin. Colloid Interface Sci. 2004, 9, 1−18.
Hofmeister effects: interplay of hydration, nonelectrostatic potentials, and ion size
Phys. Chem. Chem. Phys. 2011 13 12352 12367Parsons, D. F.; Boström, M.; Lo Nostro, P.; Ninham, B. W. Hofmeister effects: interplay of hydration, nonelectrostatic potentials, and ion size. Phys. Chem. Chem. Phys. 2011, 13, 12352−12367.
What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents.
Chem. Sci. 2017 8 7052 7065Mazzini, V.; Craig, V. S. J. What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents. Chem. Sci. 2017, 8, 7052−7065.
Volcano plots emerge from a sea of nonaqueous solvents: the law of matching water affinities extends to all solvents
ACS Cent. Sci. 2018 4 1056 1064Mazzini, V.; Craig, V. S. J. Volcano plots emerge from a sea of nonaqueous solvents: the law of matching water affinities extends to all solvents. ACS Cent. Sci. 2018, 4, 1056−1064.
Hofmeister effects at low salt concentration due to surface charge transfer
Curr. Opin. Colloid Interface Sci. 2016 23 41 49Parsons, D. F.; Salis, A. Hofmeister effects at low salt concentration due to surface charge transfer. Curr. Opin. Colloid Interface Sci. 2016, 23, 41−49.
Importance of accurate dynamic polarizabilities for the ionic dispersion interactions of alkali halides
Langmuir 2010 26 1816 1823Parsons, D. F.; Ninham, B. W. Importance of accurate dynamic polarizabilities for the ionic dispersion interactions of alkali halides. Langmuir 2010, 26, 1816−1823.
Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited
Chem. Soc. Rev. 2014 43 7358 7377Salis, A.; Ninham, B. W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358−7377.
Specific anion effects on glass electrode pH measurements of buffer solutions: bulk and surface phenomena
J. Phys. Chem. B 2006 110 2949 2956Salis, A.; Pinna, M. C.; Bilaničová, D.; Monduzzi, M.; Lo Nostro, P.; Ninham, B. W. Specific anion effects on glass electrode pH measurements of buffer solutions: bulk and surface phenomena. J. Phys. Chem. B 2006, 110, 2949−2956.
Specific ion effects on adsorption of lysozyme on functionalized SBA-15 mesoporous silica
J. Phys. Chem. B 2010 114 7996 8001Salis, A.; Bhattacharyya, M. S.; Monduzzi, M. Specific ion effects on adsorption of lysozyme on functionalized SBA-15 mesoporous silica. J. Phys. Chem. B 2010, 114, 7996−8001.
Hofmeister effect on thermo-responsive poly(N-isopropylacrylamide) hydrogels grafted on macroporous poly(vinyl alcohol) formaldehyde sponges
Chinese J. Polym. Sci. 2020 38 257 267Shi, K.; Sha, D.; Xu, J. D.; Yang, X.; Wang, B. L.; Pan, Y. X.; Ji, X. L. Hofmeister effect on thermo-responsive poly(N-isopropylacrylamide) hydrogels grafted on macroporous poly(vinyl alcohol) formaldehyde sponges. Chinese J. Polym. Sci. 2020, 38, 257−267.
Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series
J. Am. Chem. Soc. 2005 127 14505 14510Zhang, Y. J.; Furyk, S.; Bergbreiter, D. E.; Cremer, P. S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505−14510.
Guanidinium can both cause and prevent the hydrophobic collapse of biomacromolecules
J. Am. Chem. Soc. 2017 139 863 870Heyda, J.; Okur, H. I.; Hladílková, J.; Rembert, K. B.; Hunn, W.; Yang, T. L.; Dzubiella, J.; Jungwirth, P.; Cremer, P. S. Guanidinium can both cause and prevent the hydrophobic collapse of biomacromolecules. J. Am. Chem. Soc. 2017, 139, 863−870.
Effect of counterion binding to swelling of polyelectrolyte brushes
Langmuir 2021 37 5554 5562Ji, C. D.; Zhou, C.; Zhao, B. T.; Yang, J. F.; Zhao, J. Effect of counterion binding to swelling of polyelectrolyte brushes. Langmuir 2021, 37, 5554−5562.
Anion-specific effects on the behavior of pH-sensitive polybasic brushes
Langmuir 2015 31 3707 3717Willott, J. D.; Murdoch, T. J.; Humphreys, B. A.; Edmondson, S.; Wanless, E. J.; Webber, G. B. Anion-specific effects on the behavior of pH-sensitive polybasic brushes. Langmuir 2015, 31, 3707−3717.
Interactions between polyelectrolyte brushes and Hofmeister ions: chaotropes versus kosmotropes
Langmuir 2015 31 10461 10468Kou, R.; Zhang, J.; Wang, T.; Liu, G. M. Interactions between polyelectrolyte brushes and Hofmeister ions: chaotropes versus kosmotropes. Langmuir 2015, 31, 10461−10468.
Effect of anions on the cloud point temperature of aqueous poly(2-ethyl-2-oxazoline) solutions
J. Phys. Chem. B 2012 116 14510 14514Tatar Güner, P.; Demirel, A. L. Effect of anions on the cloud point temperature of aqueous poly(2-ethyl-2-oxazoline) solutions. J. Phys. Chem. B 2012, 116, 14510−14514.
Effects of Hofmeister anions on the aggregation behavior of PEO-PPO-PEO triblock copolymers
Langmuir 2011 27 9203 9210Deyerle, B. A.; Zhang, Y. J. Effects of Hofmeister anions on the aggregation behavior of PEO-PPO-PEO triblock copolymers. Langmuir 2011, 27, 9203−9210.
Counterion specificity of polyelectrolyte brushes: role of specific ion-pairing interactions
ChemPhysChem 2018 19 1404 1413Kou, R.; Zhang, J.; Chen, Z.; Liu, G. M. Counterion specificity of polyelectrolyte brushes: role of specific ion-pairing interactions. ChemPhysChem 2018, 19, 1404−1413.
Catch and release of DNA in coacervate-dispersed gels
Macromol. Rapid Commun. 2006 27 1242 1246Ohsugi, A.; Furukawa, H.; Kakugo, A.; Osada, Y.; Gong, J. P. Catch and release of DNA in coacervate-dispersed gels. Macromol. Rapid Commun. 2006, 27, 1242−1246.
Thermo-sensitive microgels supported gold nanoparticles as temperature-mediated catalyst
Chinese J. Polym. Sci. 2019 37 235 242Zhou, X. J.; Lu, H. P.; Kong, L. L.; Zhang, D.; Zhang, W.; Nie, J. J.; Yuan, J. Y.; Du, B. Y.; Wang, X. P. Thermo-sensitive microgels supported gold nanoparticles as temperature-mediated catalyst. Chinese J. Polym. Sci. 2019, 37, 235−242.
Synthesis and characterization of temperature-sensitive cellulose-graft-poly(N-isopropylacrylamide) copolymers
Chinese J. Polym. Sci. 2015 33 1640 1649Yang, L. L.; Zhang, J. M.; He, J. S.; Zhang, J.; Gan, Z. H. Synthesis and characterization of temperature-sensitive cellulose-graft-poly(N-isopropylacrylamide) copolymers. Chinese J. Polym. Sci. 2015, 33, 1640−1649.
Diethanol ammonium-borate based polybetaine with tunable UCST phase transition
Chinese J. Polym. Sci. 2016 34 777 784Shi, M.; Duan, X. R.; Liu, Z. T.; Liu, Z. W.; Jiang, J. Q. Diethanol ammonium-borate based polybetaine with tunable UCST phase transition. Chinese J. Polym. Sci. 2016, 34, 777−784.
Specific anion effects on charged-neutral random copolymers: interplay between different anion-polymer interactions
Langmuir 2021 37 1697 1706Lian, L. L.; Liu, L. D.; Ding, Y. W.; Hua, Z.; Liu, G. M. Specific anion effects on charged-neutral random copolymers: interplay between different anion-polymer interactions. Langmuir 2021, 37, 1697−1706.
Synthesis and solution properties of zwitterionic polymers
Chem. Rev. 2002 102 4177 4189Lowe, A. B.; McCormick, C. L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177−4189.
Ion-specific conformational behavior of polyzwitterionic brushes: exploiting it for protein adsorption/desorption control
Langmuir 2013 29 6588 6596Wang, T.; Wang, X. W; Long, Y. C.; Liu, G. M.; Zhang, G. Z. Ion-specific conformational behavior of polyzwitterionic brushes: exploiting it for protein adsorption/desorption control. Langmuir 2013, 29, 6588−6596.
Anion specificity of polyzwitterionic brushes with different carbon spacer lengths and its application for controlling protein adsorption
Langmuir 2016 32 2698 2707Wang, T.; Kou, R.; Liu, H. L.; Liu, L. D.; Zhang, G. Z.; Liu, G. M. Anion specificity of polyzwitterionic brushes with different carbon spacer lengths and its application for controlling protein adsorption. Langmuir 2016, 32, 2698−2707.
Anion specificity in dimethyl sulfoxide-water mixtures exemplified by a thermosensitive polymer
J. Phys. Chem. B 2018 122 8293 8300Zhu, R. W.; Baraniak, M. K.; Jäkle, F.; Liu, G. M. Anion specificity in dimethyl sulfoxide-water mixtures exemplified by a thermosensitive polymer. J. Phys. Chem. B 2018, 122, 8293−8300.
Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process
Methods 2004 34 300 311Collins, K. D. Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process. Methods 2004, 34, 300−311.
Specific ion effects in colloidal and biological systems
Curr. Opin. Colloid Interface Sci. 2010 15 34 39Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34−39.