INTRODUCTION
Polymer solar cells (PSCs) have received tremendous attention because of their great merits of solution processability with low cost, flexiblity and semi-transparency.[1−7] The mainstream PSCs use blends of polymer electron donor and small molecular electron acceptor as active layers. Another imporant kind of PSCs is all polymer solar cells (APSCs), which use blends of polymer electron donors and polymer electron acceptors as photoactive layers.[8−12] Compared with typical PSCs, APSCs have the great merits of excellent morphological stability and mechanical robustness.[21−25] Great efforts have been dovoted to the development of new polymer acceptors and optimization of polymer donor/polymer acceptors blend morphology.[26−31] As a result, in the past decade, power conversion efficiency (PCE) of APSCs has been dramatically enhanced and has reached the 15% milestone.[32−35] APSCs have emerged as a promising kind of photovoltaic technology.
Number-average molecular weight (Mn) of polymer materials plays an important role in active layer morphology and photovoltaic performance of PSCs. In typical PSCs employing polymer donor/small molecular acceptor blends, effects of Mn on active layer morphology and photovoltaic performance have been intensively studied.[36−38] In comparison, for APSCs, effects of Mn on polymer donor/polymer acceptor blend morphology are less understood. Morphology optimization of polymer donor/polymer acceptor blend is very different from that of polymer donor/small molecular acceptor blend. In the former case, polymer donor/polymer acceptor blend morphology optimization is always carried out by controlling the pre-aggregation tendency of polymer chains in solution.[39,40] This strategy has been successfully performed by changing the solvent, optimizing the solution temperature, dissolving the two polymers individually, etc.[41−43] Optimizing morphology of APSCs by controlling Mn of either polymer donors or polymer acceptors has been reported by several research groups.[44−47] Increasing Mn leads to enhanced aggregation tendency of polymer chains in solution. However, its effects on active layer morpholgy and device performance of APSCs are different case by case. Moreover, as there are both polymer donors and polymer acceptors in active layer of APSCs, the interplay of Mns of the two polymers is rarely studied. To date, only Marks et al. have optimized active layer morphology of APSCs by tuning Mns of both polymer donor and polymer acceptor. Unfortunately, the APSC device performce is moderate, i.e. PCE<5%.[48,49] Therefore, it is important to study the interplay of Mn of both polymer donor and polymer acceptor on active layer morphology of high-performance APSCs.
In this study, we select a combination of polymer donor and polymer acceptor, which can achieve high PCE of 10% in APSCs. We systematically study the effect of Mn of the two polymers on active layer morphology and photovoltaic performance of APSCs and get several important conclusions. To obtain biscontinuous fibrous network morphology and give excellent APSC device performance, either the polymer donor or the polymer acceptor or both should have high Mn. When the Mn of the polymer acceptor is high, the APSC device performance is insensitive to the Mn of the other polymer. The optimal APSC device performance is obtained when the Mns of both the polymer donor and the polymer acceptor are medium. This result provides a comprehensive understanding on the matching of Mn of polymer donors and polymer acceptors in high-performance APSCs.
EXPERIMENTAL
Materials
Poly[4-(5-(4,8-bis(5-((2-butyloctyl)thio)thiophen-2-yl)-6-methylbenzo[1,2-b:4,5-b']dithiophen-2-yl)thiophen-2-yl)-5,6-difluoro-2-(2-hexyldecyl)-7-(5-methylthiophen-2-yl)-2H-benzo[d][1,2,3]triazole] (CD1) and poly[4-(5-(5,10-bis(2-dodecylhexadecyl)-4,4,9,9-tetrafluuoro-7-methyl-4,5,9,10-tetrahydro3a,5,8,10-tetraaza-4,9-diborapyren-2-yl)thiophen-2-yl)-7-(5-methylthiophen-2-yl)benzo[c][1,2,5]thiadiazole] (PBN-14) with different Mns were synthesized in the laboratory according our previous reported method.[50,51]
APSC Devices Fabrication
APSC devices were fabricated with the configuration of ITO/PEDOT:PSS/active layer/LiF/Al. ITO glass substrates were cleaned by sequential ultrasonication in detergent, deionized water, acetone, and isopropyl alcohol, followed by drying at 120 °C for 30 min and treating with UV-ozone for 25 min. Then the PEDOT:PSS solution (Clevios VP Al 4083 from H. C. Starck Inc.) was spin-coated on the ITO glass substrates at 5000 r/min for 40 s to give a thickness of 40 nm, and baked at 120 °C for 30 min. The PEDOT:PSS substrates were transferred to a nitrogen-filled glove box. CD1 and PBN-14 with a weight ratio of 1.5:1 were dissolved together in chlorobenzene (CB) with a total concentration of 10 mg/mL. The solution was stirred at 90 °C for 6 h and not cooling, and then the active layer was hot spin coating. The films thickness was maintained in the range of 80−100 nm. The active layers were annealed at 100 °C for 10 min, after that, 0.5 nm LiF and 100 nm Al were thermally deposited onto the active layer through a shadow mask in a vacuum chamber with a base pressure of 2×10−4 Pa. The effective area of the device was defined to be 0.08 cm2, which was further confined as 0.02 cm2 by a non-refractive mask to improve the accuracy of measurements.
Hole- and Electron-only Devices Fabrication and Mobility Measurements
The hole and electron mobilities were measured by SCLC me thod. The hole-only device structure is ITO/PEDOT:PSS (40 nm)/active layer/MoO3 (10 nm)/Al (100 nm) and the electron-only device structure is ITO/PEIE (10 nm)/active layer/Ca (20 nm)/Al (100 nm), respectively. J-V plots in the range of 0–10 V were measured using a Keithley 2400 source meter, and the mobility was obtained by fitting the J-V plot near quadratic region according to the modified Mott-Gurney equation:

where J is the current density, ε0 is permittivity of free space, εr is the relative permittivity (assumed to be 3), μ is the zero-field mobility, V is the potential across the device (V = Vapplied − Vbi − Vseries), d is the thickness of active layer. The series and contact resistance of the device (10–20 Ω) were measured using blank device of ITO/PEDOT:PSS/MoO3/Al or ITO/PEIE/Ca/Al.
Characterization
The J-V plots of the APSC devices were measured using a Keithley 2400 source meter under 100 mW/cm2 AM 1.5G simulated solar light illumination provided by a XES-40S2-CE Class Solar Simulator (Japan, SAN-EI Electric Co., Ltd.). An aperture with an area of 2 mm2 was used to accurately measure the device performance. The EQE spectra were measured using a Solar Cell Spectral Response Measurement System QE-R3011 (Enlitech Co., Ltd.) under the short-circuit condition at a chopping frequency of 165 Hz. Temperature-dependent UV-Vis absorption spectra of different Mn CD1 and PBN-14 in solution were measured with a Perkin-Elmer Lambda 35 UV-Vis spectrometer. The solution samples were prepared using chlorobenzene solvent with a concentration of 0.1 mg/mL. The transmission electron microscopy (TEM) measurement was performed on a JEOL JEM-1400 transmission electron microscope operating at 120 kV. The atomic force microscopy (AFM) characterization was performed on a SPA 300HV instrument with a SPI 3800 controller (Seiko Instruments). A silicon micro cantilever (spring constant 2 N/m and resonance frequency ca. 300 kHz, Olympus Co., Japan) with an etched conical tip was used for the scan. The thickness of films was measured with a XP-plus Stylus Profilometer. Two-dimensional grazing-incidence wide angle X-ray scattering (2D-GIWAXS) was measured at Shanghai Synchrotron Radiation Facility (SSRF) on beam line BL14B1 (λ=0. 124 nm) with a MarCCD area detector at incidence angle of 0.16°. Samples were prepared on Si substrates using identical blend solutions as those used in devices.
RESULTS AND DISCUSSION
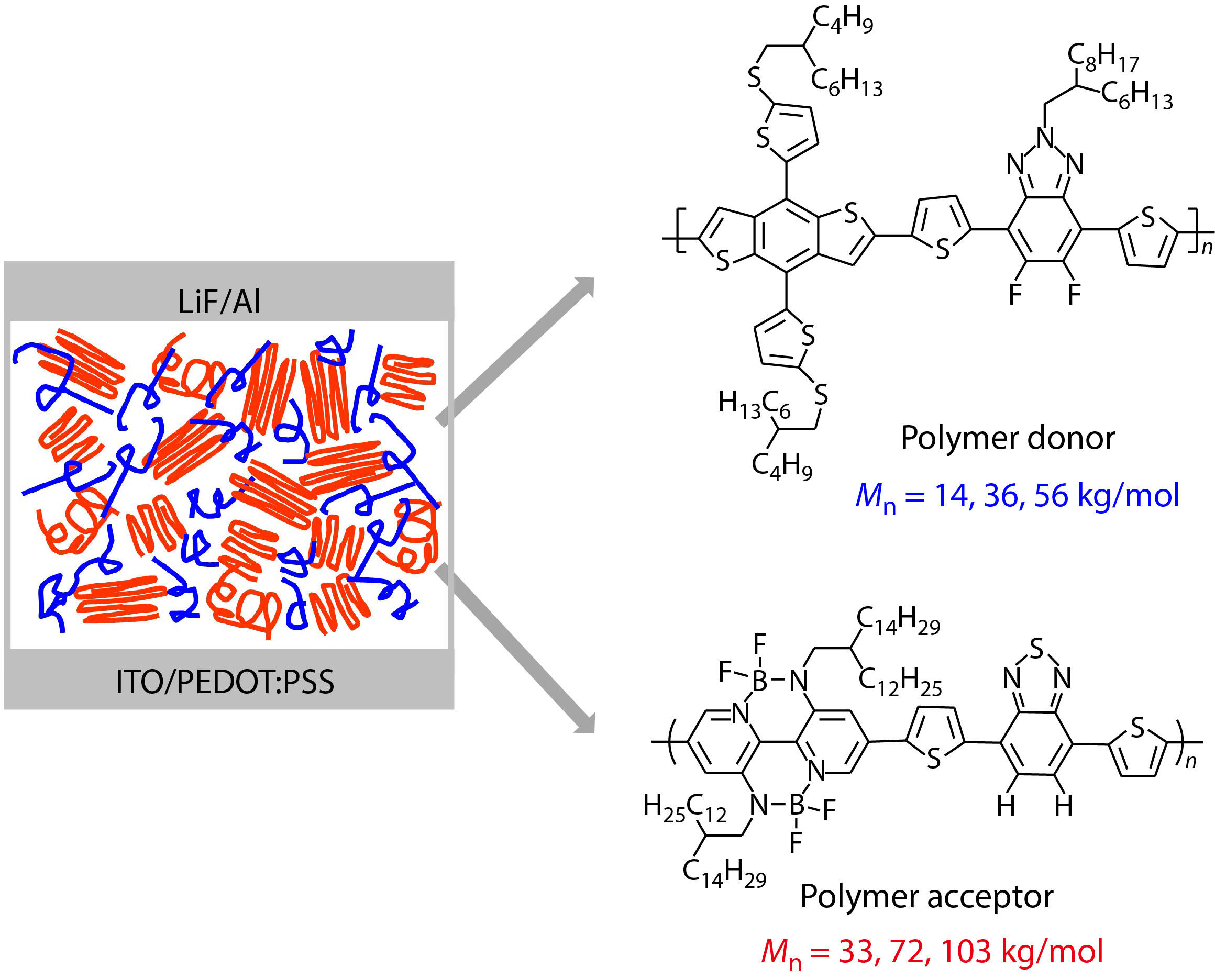
As shown in Fig. 1, we select CD1 as the polymer donor and PBN-14 as the polymer acceptor. The opto-electronic properties of CD1 and PBN-14 have been previously reported by us.[50,51] Both CD1 and PBN-14 are semi-crystalline in thin film.
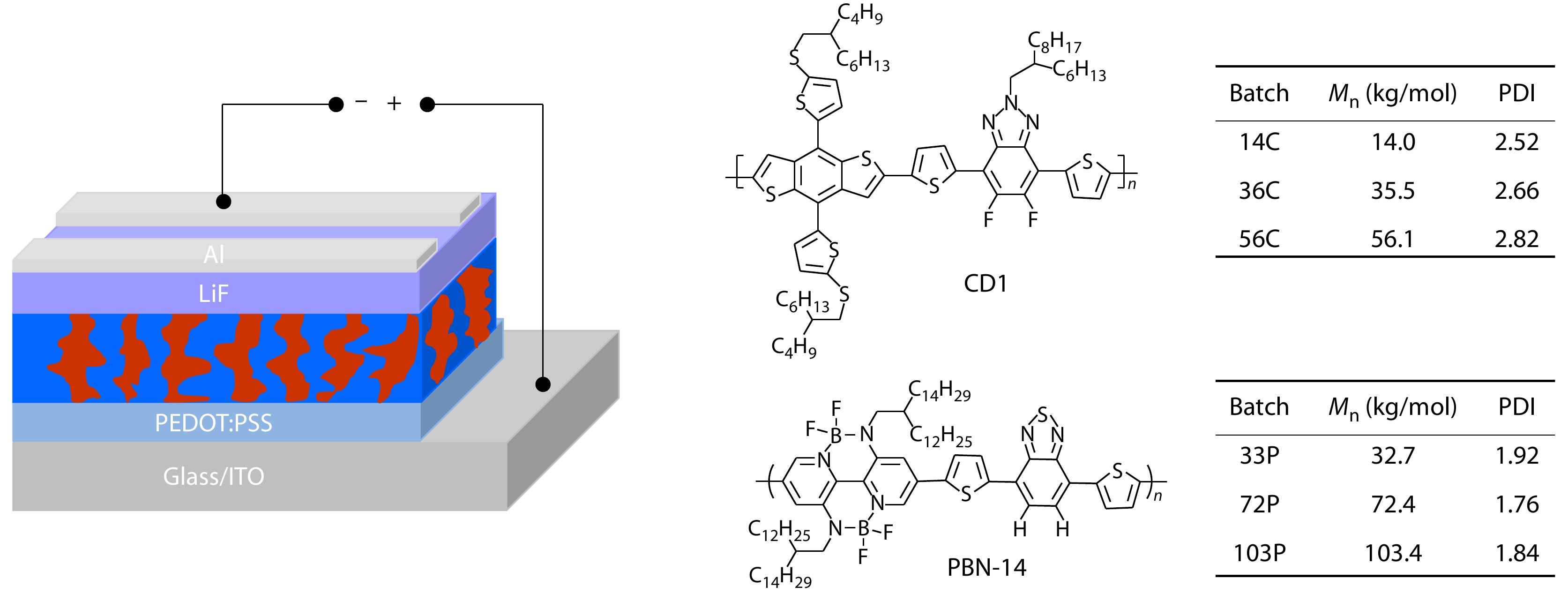
In this work, we synthesized CD1 and PBN-14 with various Mns by controlling the polymerization time. We obtained CD1 with the Mn of 14.0, 35.5 and 56.1 kg/mol, which were named as 14C, 36C and 56C respectively. Three batches of PBN-14 with the Mns of 32.7, 72.4 and 103.4 kg/mol were also synthesized and named as 33P, 72P and 103P, respectively. The polydispersity index (PDI) of CD1 and PBN-14 of various batches is similar (see Fig. 1).
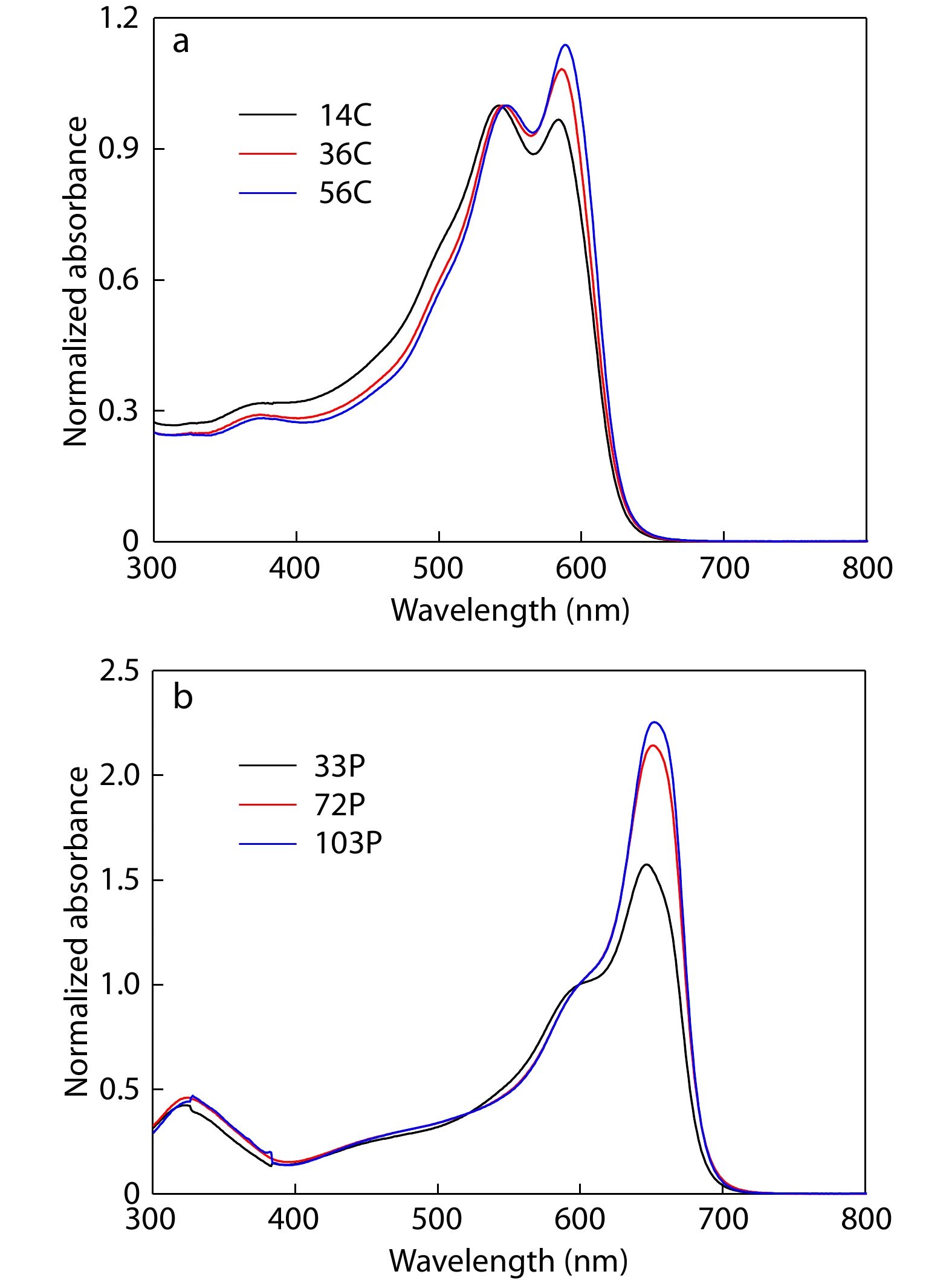
Aggregation tendencies of polymer donors and polymer acceptors in solution greatly affect blend morphology of active layers in APSCs.[52,53] The aggregation tendency was studied by the UV-Vis absorption spectra of the polymers in solution. Figs. 2(a) and 2(b) show the UV-Vis spectra of CD1 and PBN-14 with different Mns in chlorobenzene solution, respectively. In the absorption spectra of CD1, the ratio of the two absorption peaks can be used to estimate the aggregation tendency of the polymer chains in solution. With the increase of Mn, the relative intensity of the long-wavelength peak at 584 nm increases, indicating stronger aggregation tendency of CD1 polymer chains in chlorobenzene solution. Similar trend is also observed in the absorption spectra of PBN-14 with different Mns. The increased Mn of PBN-14 also leads to stronger aggregation tendency of PBN-14 polymer chains in solution. The strong aggregation tendency in solution at high Mn is expected to lead to excellent phase separation morphology of active layers of APSCs.
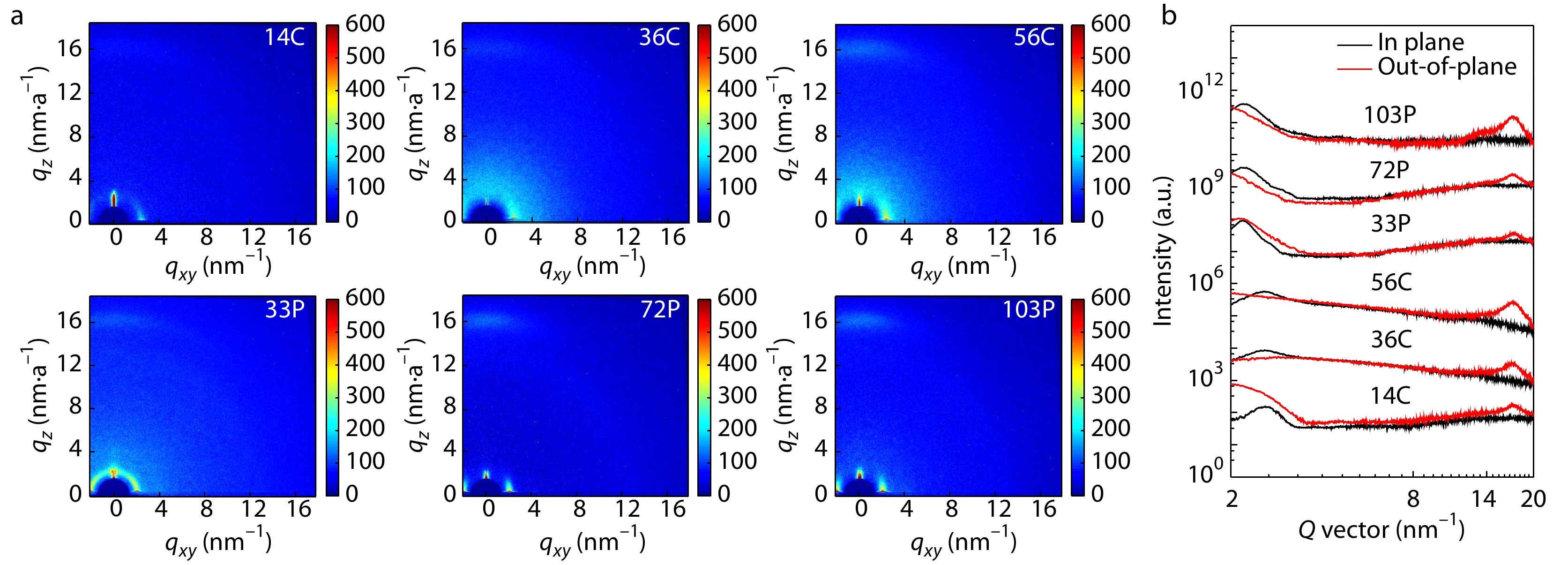
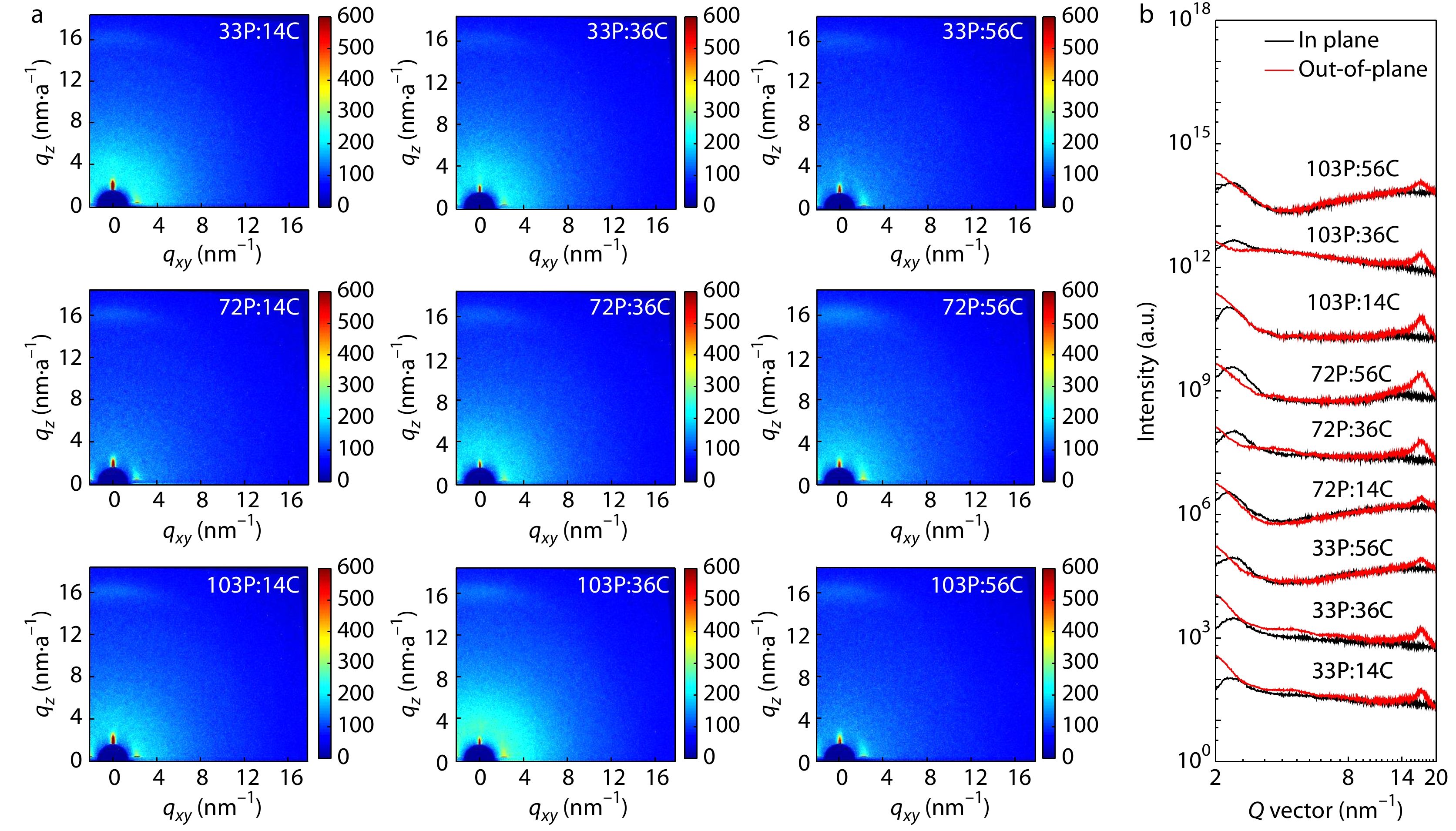
Two-dimensional grazing incidence wide-angle X-ray scattering (2D-GIWAXS) was utilized to characterize the packing of polymer chains of CD1 and PBN-14 in thin film. The 2D-GIWAXS patterns of the polymer films are shown in Fig. 3 and the corresponding data of the GIWAXS patterns are summarized in Table S3 (in the electronic supplementary information, ESI). All the six CD1 and PBN-14 films exhibit the (100) peak mainly in the in-plane direction and the (010) peak in the out-of-plane direction, indicating face-on orientation of the polymer chains in thin film. The Mns of CD1 and PBN-14 do not affect the orientation of the polymer chains in thin film. We estimate the average crystallization size of the films by calculating the coherence length (CL) of the (100) reflection peak in the in-plane direction and the (010) reflection peak in the out-of-plane direction using the Scherrer equation. The CL values of the (100) peaks of 14C are obviously larger than those of 36C and 56C. The CL values of the (100) peaks of 33P are also larger than those of 72P and 103P. These results probably suggest that high Mn leads to suppressed crystallinity in thin film.
We measured the hole mobility (μh) of the three CD1 samples and the electron mobility (μe) of the three PBN-14 samples using space charge limited current (SCLC) method with the hole-only devices and the electron-only devices, respectively.[54,55] The current denstiy-voltage (J-V) curves of the devices are shown in Fig. S4 (in ESI). The μh and μe values are listed in Table S1 (in ESI). With the increasing Mn, the μh of CD1 gradually increases, the μe of PBN-14 increases first and then decreases. The three CD1 samples of different Mns show similar absorption spectra in thin film and similar LUMO/HOMO energy levels. Similar trend is also observed for the three PBN-14 samples. These results indicate that the Mns of the polymers do not affect the energy levels but affect the charge transporting properties.
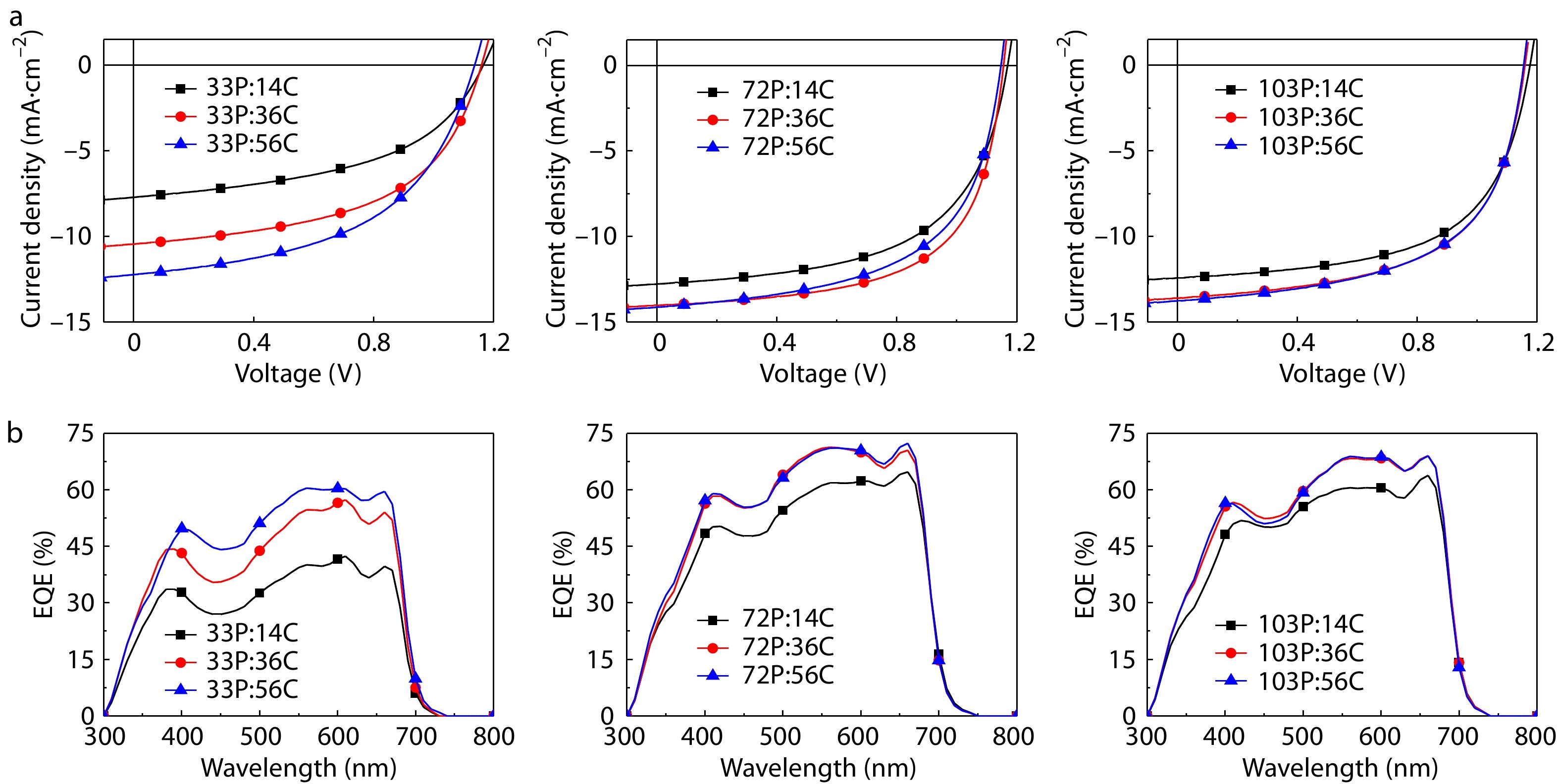
APSCs devices with the conventional configuration of ITO/PEDOT:PSS/CD1:PBN-14/LiF/Al were fabricated to investigate the effect of molecualr weights on device performance. CD1 and PBN-14 were dissolved together in chlorobenzene (CB) with a weight ratio of 1.5:1 and a total concentration of 10 mg/mL. The active layer was spin-coated with the solution of CD1 and PBN-14 at 90 °C, followed by thermal annealing at 100 °C for 10 min. The J-V curves of the APSCs devices fabricated with various Mn combinations are shown in Fig. 4(a) and the detailed parameters are collected in Table 1. The nine devices exhibit similar open circuit voltage (VOC), different short-circuit current density (JSC) and fill factor (FF). The PCE varies greatly from 4.43% to 10.06%, indicating that Mn of the polymer donors and polymer acceptors play an important role in the APSC device performance. The nearly unaffected VOC is attributed to the little effect of Mn on LUMO/HOMO energy levels of the polymers. The different JSC and FF values are due to the phase separation morphology of the active layers. Fig. 4(b) shows the external quantum efficiency (EQE) curves of the device. The EQE spectra are in accordance with the dependence of the integrated JSC value on different Mn donor-acceptor combinations.
| Acceptor | Donor | VOC (V) | JSC (mA·cm–2) | Cal. JSC (mA·cm–2) | FF (%) | PCE a (%) |
| a The average parameters were calculated from 10 devices. | ||||||
| 33P | 14C | 1.17 (1.17 | 7.72 (7.45 | 7.40 | 49.1 (48.0 | 4.43 (4.05 |
| 36C | 1.16 (1.16 | 10.44 (10.23 | 10.12 | 53.0 (52.2 | 6.42 (6.21 | |
| 56C | 1.14 (1.14 | 12.22 (11.91 | 11.96 | 51.0 (50.0 | 7.10 (6.75 | |
| 72P | 14C | 1.17 (1.17 | 12.77 (12.50 | 12.43 | 57.5 (56.1 | 8.59 (8.18 |
| 36C | 1.16 (1.16 | 14.03 (13.64 | 13.75 | 61.8 (60.5 | 10.06 (9.69 | |
| 56C | 1.15 (1.15 | 14.12 (13.76 | 13.83 | 57.8 (57.1 | 9.39 (9.02 | |
| 103P | 14C | 1.18 (1.18 | 12.42 (12.11 | 12.17 | 59.4 (58.2 | 8.70 (8.41 |
| 36C | 1.16 (1.16 | 13.59 (13.35 | 13.34 | 59.1 (58.4 | 9.32 (9.04 | |
| 56C | 1.16 (1.16 | 13.76 (13.57 | 13.42 | 58.2 (57.4 | 9.29 (9.01 | |
By comparing the PCE of the nine APSCs devices, we analyze the interplay of the Mn of CD1 and PBN-14 on their effect on the device performance. No matter what the Mn of the polymer acceptor is, the PCEs of the devices of 14C are always lower than those of the corresponding devices of 36C and 56C. Moreover, no matter what Mn of the polymer donor is, the PCEs of the devices of 33P are lower than those of 72P and 103P. According to these results, we believe that sufficiently high Mn of both polymer donor and polymer acceptor are necessary to obtain optimal APSC device peformance. With 33P as the polymer acceptor, the PCE of the devices increases greatly from 4.43% to 7.10% when the Mn of polymer donors increases from 14C to 56C. In comparison, with 72P or 103P as the polymer acceptor, the PCE of the devices are only weakly dependent on the Mn of the polymer donors. The higher Mn of the polymer accptor, the weaker dependence of PCE on the Mn of the polymer donors. Among the nine devices, the highest PCE of 10.06% is obtained with the 72P:36C blend, indicating that medium Mn of both the polymer donor and the polymer acceptor are required for the optimal APSC device performance.
To elucidate the reason behind the effect of Mn on photovoltaic performance, we studied the active layer morphology of the APSCs devices by 2D-GIWAXS, atomic force microscopy (AFM) and transmissions electron microscopy (TEM). The 2D-GIWAXS patterns of the active layers are shown in Fig. 5 and the corresponding data are summarized in Table 2. All the active layers exhibit the (100) diffraction peak at ca. 0.24 Å–1 and the (010) diffraction peak at 1.70 Å–1. Both the (100) peak and the (010) peak are arributed to the sum of the polymer donor and the polymer acceptor. The (100) peak in the in-plane direction and the (010) peak in the out-of-plane direction suggest that the polymer donor and the polymer acceptor both adopt face-on orientation in the active layers. The Mn does not affect the face-on/edge-on orientation of the polymer donor and the polymer acceptor. As the Mn of CD1 increases, the CL values of the (100) peak and the (010) peak gradually decrease, suggesting decreasing crystallinity of the polymers in the blend films. This trend exists for the donor/acceptor combinations with all the three polymer acceptors. For the dependence of CL values on the Mn of polymer acceptors, no clear correlation can be observed. Among the nine active layers, the one of 72P:36C blend with the best photovoltaic performance shows medium CL values of both the (100) peak and the (010) peak.
| Film | 100 (In-plane direction) | 010 (Out-of-plane direction) | |||||||
| Location (Å–1) | d-Spacing (Å) | FWHM (Å–1) | CL (Å) | Location (Å–1) | d-Spacing (Å) | FWHM (Å–1) | CL (Å) | ||
| 33P:14C | 0.24 | 25.75 | 0.069 | 82 | 1.70 | 3.69 | 0.171 | 33 | |
| 33P:36C | 0.25 | 25.54 | 0.071 | 77 | 1.70 | 3.69 | 0.186 | 30 | |
| 33P:56C | 0.23 | 26.97 | 0.090 | 63 | 1.70 | 3.69 | 0.219 | 26 | |
| 72P:14C | 0.23 | 27.56 | 0.066 | 86 | 1.71 | 3.68 | 0.187 | 30 | |
| 72P:36C | 0.24 | 25.75 | 0.069 | 82 | 1.70 | 3.69 | 0.195 | 29 | |
| 72P:56C | 0.24 | 26.29 | 0.072 | 79 | 1.70 | 3.69 | 0.227 | 25 | |
| 103P:14C | 0.24 | 26.62 | 0.059 | 96 | 1.70 | 3.69 | 0.177 | 32 | |
| 103P:14C | 0.24 | 25.96 | 0.078 | 72 | 1.70 | 3.69 | 0.184 | 31 | |
| 103P:36C | 0.24 | 26.51 | 0.083 | 68 | 1.70 | 3.69 | 0.226 | 25 | |
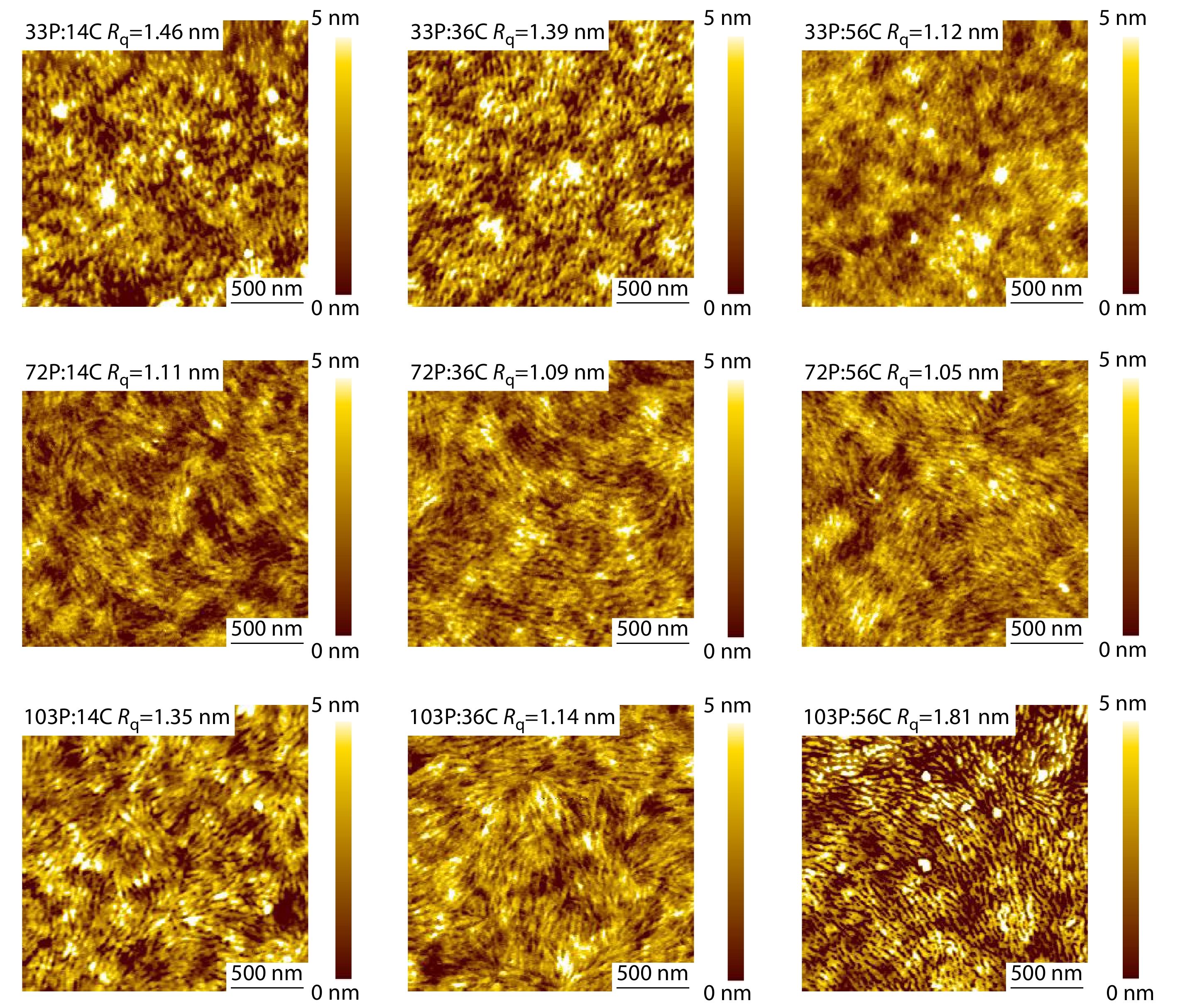
Fig. 6 shows the AFM height images of the various CD1:PBN-14 combinations. Except those of the 33P:14C blend and the 33P:36C blend, the surfaces of the other seven active layers all show bicontinuous fiberous network microstructure. The fiberous microstructure are beneficial for exciton diffusion, exciton dissociation and charge transporting.[56−59] The AFM height image results are consistent with the poor photovoltaic performance of the 33P:14C and 33P:36C blends as well as the excellent photovoltaic performance of the other seven active layers. The higher Mn of either the polymer donor or the polymer acceptor, the more surface microstructures with thin, long and bicontinuous morphologies. This result indicates that high Mn is dominant for the bicontinuous fiberous network microstructure of the active layer of APSCs. The root-mean-square roughness (Rq) values of the AFM images are also shown in Fig. 6. Obviously, with the increasing Mn of the polymer donor, the Rq values decreases. An exception is the 103P:56C blend, which exhibits rough surface with large Rq value of 1.86 nm. When the Mn of PBN-14 is 72P, all the three active layers with various Mns of CD1 show smooth surface with small Rq values.
Based on the above result on active layer morphology, we analyze the effects of Mn on phase separation morphology of polymer donor/polymer acceptor blends. High Mn plays an important role in the formation of bicontinuous fibrous network morphology of active layers of APSCs. When both CD1 and PBN-14 have low Mn, the active layer morphology is poor and the PCE is low. At least one polymer should have high Mn to ensure the bicontinuous fibrous network morphology. When the Mn of PBN-14 is high, the resulting active layers all exhibit bicontinuous fibrous network morphology and the PCE of the APSC devices is insensitive to the Mn of CD1. Probably, the aggregation and crystallization of PBN-14 play a more important role in the phase separation of the active layers than those of CD1. This maybe is due to the stronger aggregation tendency in solution and the higher Mn on-general of PBN-14 than those of CD1.
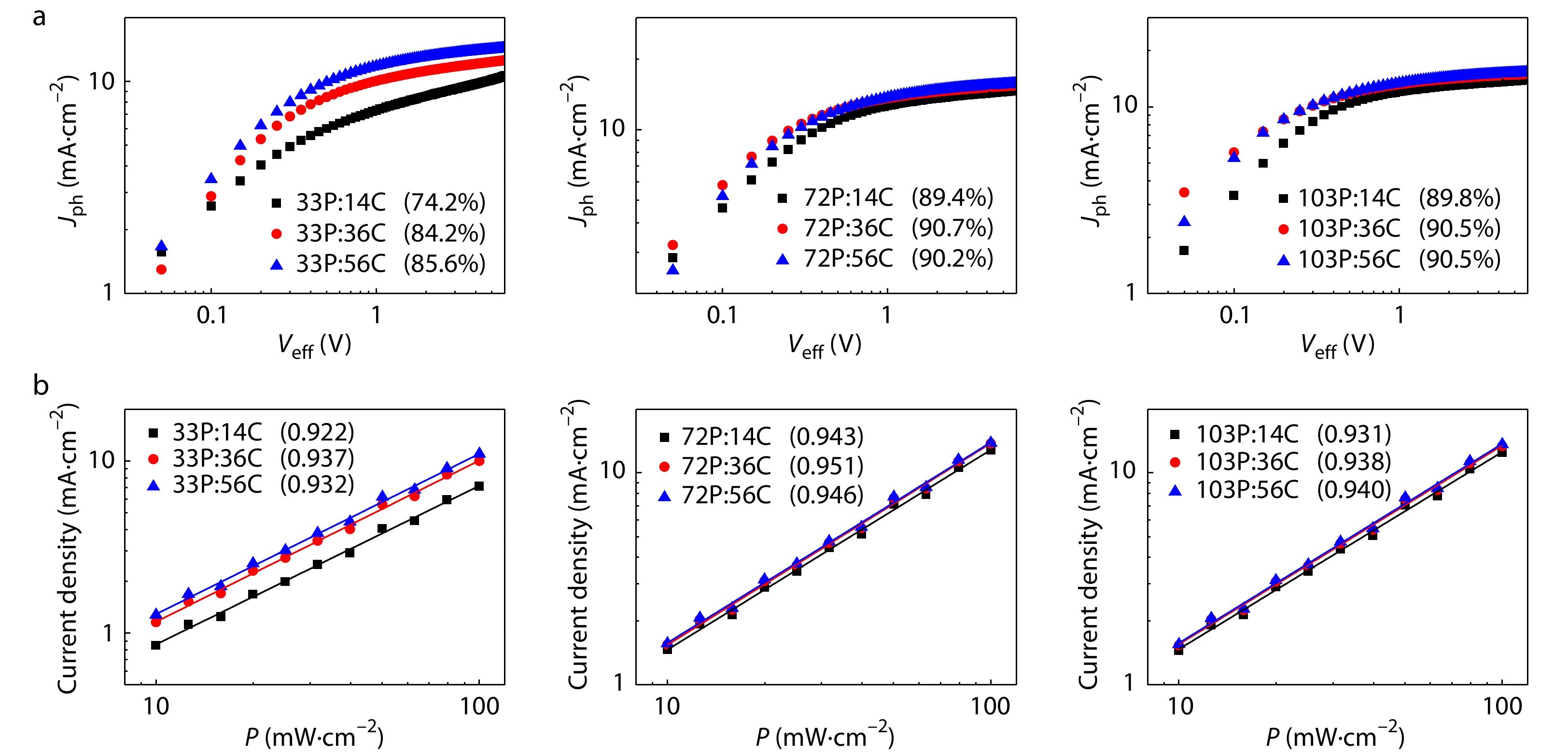
To understand the reason for the difference of the APSC device performance, we studied the charge transport, collection, and recombination behaviors of the devices. The hole and electron mobilities of the devices were estimated by SCLC method with the J-V plots in dark of the hole-only and the electron-only devices. Fig. S5 (in ESI) shows the J-V curves and Table S2 (in ESI) lists the hole and electron mobilities. Irrespective of the Mn of PBN-14, as the Mn of CD1 increases, the μh of the blend films gradually increases. However, the dependence of μe of the blend films on the Mn of CD1 is different at various Mns of PBN-14. This is attributed to the improved formation of continueous networks of CD1 phase in the blend films at high Mn. The formation of continueous networks of CD1 phase also affects the networks of PBN-14 phase. To study the exciton dissociation and charge collection probabilities of the APSC devices, we measured the dependence of photocurrent density (Jph) on effective voltage (Veff) of the devices. From the Jph-Veff curves as shown in Fig. 7(a), saturated photocurrent density (Jsat) and charge collection probabilities (Jph/Jsat) can be estimated. When the Mn of the acceptor PBN-14 is high (72P and 103P), all the devices exhibit high charge collection probability of about 90%. When the polymer acceptor is 33P with low Mn, as the Mn of the polymer donor CD1 increases, the Jph/Jsat gradually increases, implying increasing charge collection probability. We measured the dependence of JSC on light intensity (I) of the APSC devices to evaluate the bimolecular recombination behaviors. The results are shown in Fig. 7(b). The relationship between JSC and I is defined as JSC
CONCLUSIONS
In summary, we systematically study the effects of Mn of the two polymers on active layer morphology and photovoltaic performance of APSCs by synthesizing a series of different Mn polymer donor CD1 and polymer acceptor PBN-14. The active layers of CD1:PBN-14 blends exhibit bicontinuous fibrous network morphololgy and achieve PCE of 10% in APSC devices. To get the desired active layer morphology of polymer donor/polymer acceptor blends, at least one polymer should have high Mn. When Mn of the polymer acceptor is high, the APSC device performance is insensitive to the Mn of the polymer donor. The optimal APSC device performance is obtained when the Mn of both the polymer donor and the polymer acceptor are medium. These results provide a comprehensive and deep understanding of the matching of Mn of polymer donors and polymer acceptors in high-performance APSCs.
Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor-acceptor heterojunctions
Science 1995 270 1789 1791Yu, G.; Gao, J.; Hummelen, J. C.; Wudl, F.; Heeger, A. J. Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789−1791.
Nonfullerene acceptor molecules for bulk heterojunction organic solar cells
Chem. Rev. 2018 118 3447 3507Zhang, G.; Zhao, J.; Chow, P. C. Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene acceptor molecules for bulk heterojunction organic solar cells. Chem. Rev. 2018, 118, 3447−3507.
Organic solar cells based on non-fullerene acceptors
Nat. Mater. 2018 17 119 128Hou, J.; Inganas, O.; Friend, R. H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119−128.
Non-fullerene acceptors for organic solar cells
Nat. Rev. Mater. 2018 3 18003Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A. K. Y.; Marder, S. R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003.
Next-generation organic photovoltaics based on non-fullerene acceptors
Nat. Photonics 2018 12 131 142Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131−142.
Wide-gap non-fullerene acceptor enabling high-performance organic photovoltaic cells for indoor applications
Nature Energy 2019 4 768 775Cui, Y.; Wang, Y.; Bergqvist, J.; Yao, H.; Xu, Y.; Gao, B.; Yang, C.; Zhang, S.; Inganäs, O.; Gao, F.; Hou, J. Wide-gap non-fullerene acceptor enabling high-performance organic photovoltaic cells for indoor applications. Nature Energy 2019, 4, 768−775.
Slot-die printed non-fullerene organic solar cells with the highest efficiency of 12.9% for low-cost PV-driven water splitting
Nano Energy 2019 61 559 566Wu, Q.; Guo, J.; Sun, R.; Guo, J.; Jia, S.; Li, Y.; Wang, J.; Min, J. Slot-die printed non-fullerene organic solar cells with the highest efficiency of 12.9% for low-cost PV-driven water splitting. Nano Energy 2019, 61, 559−566.
Recent advances, design guidelines, and prospects of all-polymer solar cells
Chem. Rev. 2019 119 8028 8086Lee, C.; Lee, S.; Kim, G. U.; Lee, W.; Kim, B. J. Recent advances, design guidelines, and prospects of all-polymer solar cells. Chem. Rev. 2019, 119, 8028−8086.
Improved performance of all-polymer solar cells enabled by naphthodiperylenetetraimide-based polymer acceptor
Adv. Mater. 2017 29 1700309Guo, Y.; Li, Y.; Awartani, O.; Han, H.; Zhao, J.; Ade, H.; Yan, H.; Zhao, D. Improved performance of all-polymer solar cells enabled by naphthodiperylenetetraimide-based polymer acceptor. Adv. Mater. 2017, 29, 1700309.
A vinylene-bridged perylenediimide-based polymeric acceptor enabling efficient all-polymer solar cells processed under ambient conditions
Adv. Mater. 2016 28 8483 8489Guo, Y.; Li, Y.; Awartani, O.; Zhao, J.; Han, H.; Ade, H.; Zhao, D.; Yan, H. A vinylene-bridged perylenediimide-based polymeric acceptor enabling efficient all-polymer solar cells processed under ambient conditions. Adv. Mater. 2016, 28, 8483−8489.
Aromatic-diimide-based n-type conjugated polymers for all-polymer solar cell applications
Adv. Mater. 2019 31 e1804699Yang, J.; Xiao, B.; Tang, A.; Li, J.; Wang, X.; Zhou, E. Aromatic-diimide-based n-type conjugated polymers for all-polymer solar cell applications. Adv. Mater. 2019, 31, e1804699.
Comparison of three n-type copolymers based on benzodithiophene and naphthalene diimide/perylene diimide/fused perylene diimides for all-polymer solar cells application
ACS Appl. Mater. Interfaces 2018 10 23263 23269Yang, J.; Yin, Y.; Chen, F.; Zhang, Y.; Xiao, B.; Zhao, L.; Zhou, E. Comparison of three n-type copolymers based on benzodithiophene and naphthalene diimide/perylene diimide/fused perylene diimides for all-polymer solar cells application. ACS Appl. Mater. Interfaces 2018, 10, 23263−23269.
Optimizing domain size and phase purity in all-polymer solar cells by solution ordered aggregation and confinement effect of the acceptor
J. Mater. Chem. C 2019 7 12560 12571Zhang, Q.; Chen, Z.; Ma, W.; Xie, Z.; Han, Y. Optimizing domain size and phase purity in all-polymer solar cells by solution ordered aggregation and confinement effect of the acceptor. J. Mater. Chem. C 2019, 7, 12560−12571.
Sequential blade-coated acceptor and donor enables simultaneous enhancement of efficiency, stability, and mechanical properties for organic solar cells
Adv. Energy Mater. 2020 10 1903609Wang, Y.; Zhu, Q.; Naveed, H. B.; Zhao, H.; Zhou, K.; Ma, W. Sequential blade-coated acceptor and donor enables simultaneous enhancement of efficiency, stability, and mechanical properties for organic solar cells. Adv. Energy Mater. 2020, 10, 1903609.
Developing conjugated polymers with high electron affinity by replacing a C-C Unit with a B←N unit
Angew. Chem. Int. Ed. 2015 54 3648 3652Dou, C. D.; Ding, Z. C.; Zhang, Z. J.; Xie, Z. Y.; Liu, J.; Wang, L. X. Developing conjugated polymers with high electron affinity by replacing a C-C Unit with a B←N unit. Angew. Chem. Int. Ed. 2015, 54, 3648−3652.
Polymer acceptor based on B←N units with enhanced electron mobility for efficient all-polymer solar cells
Angew. Chem. Int. Ed. 2016 55 5313 5317Zhao, R.; Dou, C.; Xie, Z.; Liu, J.; Wang, L. Polymer acceptor based on B←N units with enhanced electron mobility for efficient all-polymer solar cells. Angew. Chem. Int. Ed. 2016, 55, 5313−5317.
An alternating polymer of two building blocks based on B←N unit: non-fullerene acceptor for organic photovoltaics
Chinese J. Polym. Sci. 2016 35 198 206Zhao, R.; Dou, C.; Liu, J.; Wang, L. An alternating polymer of two building blocks based on B←N unit: non-fullerene acceptor for organic photovoltaics. Chinese J. Polym. Sci. 2016, 35, 198−206.
Conjugated polymers containing B←N unit as electron acceptors for all-polymer solar cells
Sci. China Chem. 2017 60 450 459Dou, C.; Liu, J.; Wang, L. Conjugated polymers containing B←N unit as electron acceptors for all-polymer solar cells. Sci. China Chem. 2017, 60, 450−459.
A Narrow-bandgap n-type polymer semiconductor enabling efficient all-polymer solar cells
Adv. Mater. 2019 31 e1905161Shi, S.; Chen, P.; Chen, Y.; Feng, K.; Liu, B.; Chen, J.; Liao, Q.; Tu, B.; Luo, J.; Su, M.; Guo, H.; Kim, M. G.; Facchetti, A.; Guo, X. A Narrow-bandgap n-type polymer semiconductor enabling efficient all-polymer solar cells. Adv. Mater. 2019, 31, e1905161.
High-performance all-polymer solar cells enabled by an n-type polymer based on a fluorinated imide-functionalized arene
Adv. Mater. 2019 31 e1807220Sun, H.; Tang, Y.; Koh, C. W.; Ling, S.; Wang, R.; Yang, K.; Yu, J.; Shi, Y.; Wang, Y.; Woo, H. Y.; Guo, X. High-performance all-polymer solar cells enabled by an n-type polymer based on a fluorinated imide-functionalized arene. Adv. Mater. 2019, 31, e1807220.
Molecular packing control enables excellent performance and mechanical property of blade-cast all-polymer solar cells
Nano Energy 2019 59 277 284Lin, B.; Zhang, L.; Zhao, H.; Xu, X.; Zhou, K.; Zhang, S.; Gou, L.; Fan, B.; Zhang, L.; Yan, H.; Gu, X.; Ying, L.; Huang, F.; Cao, Y.; Ma, W. Molecular packing control enables excellent performance and mechanical property of blade-cast all-polymer solar cells. Nano Energy 2019, 59, 277−284.
Comparative study of the mechanical properties of all-polymer and fullerene–polymer solar cells: the importance of polymer acceptors for high fracture resistance
Chem. Mater. 2018 30 2102 2111Kim, W.; Choi, J.; Kim, J. H.; Kim, T.; Lee, C.; Lee, S.; Kim, M.; Kim, B. J.; Kim, T. S. Comparative study of the mechanical properties of all-polymer and fullerene–polymer solar cells: the importance of polymer acceptors for high fracture resistance. Chem. Mater. 2018, 30, 2102−2111.
Thermally stable all-polymer solar cells with high tolerance on blend ratios
Adv. Energy Mater. 2018 8 1800029Zhang, Y.; Xu, Y.; Ford, M. J.; Li, F.; Sun, J.; Ling, X.; Wang, Y.; Gu, J.; Yuan, J.; Ma, W. Thermally stable all-polymer solar cells with high tolerance on blend ratios. Adv. Energy Mater. 2018, 8, 1800029.
Mechanically robust and high-performance ternary solar cells combining the merits of all-polymer and fullerene blends
J. Mater. Chem. A 2018 6 4494 4503Lee, W.; Kim, J. H.; Kim, T.; Kim, S.; Lee, C.; Kim, J. S.; Ahn, H.; Kim, T. S.; Kim, B. J. Mechanically robust and high-performance ternary solar cells combining the merits of all-polymer and fullerene blends. J. Mater. Chem. A 2018, 6, 4494−4503.
From fullerene-polymer to all-polymer solar cells: the importance of molecular packing, orientation, and morphology control
Acc. Chem. Res. 2016 49 2424 2434Kang, H.; Lee, W.; Oh, J.; Kim, T.; Lee, C.; Kim, B. J. From fullerene-polymer to all-polymer solar cells: the importance of molecular packing, orientation, and morphology control. Acc. Chem. Res. 2016, 49, 2424−2434.
Controlling molecular mass of low-band-gap polymer acceptors for high-performance all-polymer solar cells
Joule 2020 4 1070 1086Wang, W.; Wu, Q.; Sun, R.; Guo, J.; Wu, Y.; Shi, M.; Yang, W.; Li, H.; Min, J. Controlling molecular mass of low-band-gap polymer acceptors for high-performance all-polymer solar cells. Joule 2020, 4, 1070−1086.
Impact of isomer design on physicochemical properties and performance in high-efficiency all-polymer solar cells
Macromolecules 2020 53 9026 9033Yang, H.; Fan, H.; Wang, Z.; Yan, H.; Dong, Y.; Cui, C.; Ade, H.; Li, Y. Impact of isomer design on physicochemical properties and performance in high-efficiency all-polymer solar cells. Macromolecules 2020, 53, 9026−9033.
A narrow-bandgap n-type polymer with an acceptor-acceptor backbone enabling efficient all-polymer solar cells
Adv. Mater. 2020 32 e2004183Sun, H.; Yu, H.; Shi, Y.; Yu, J.; Peng, Z.; Zhang, X.; Liu, B.; Wang, J.; Singh, R.; Lee, J.; Li, Y.; Wei, Z.; Liao, Q.; Kan, Z.; Ye, L.; Yan, H.; Gao, F.; Guo, X. A narrow-bandgap n-type polymer with an acceptor-acceptor backbone enabling efficient all-polymer solar cells. Adv. Mater. 2020, 32, e2004183.
Fluorinated end group enables high-performance all-polymer solar cells with near-infrared absorption and enhanced device efficiency over 14%
Adv. Energy Mater. 2020 11 2003171Yu, H.; Qi, Z.; Yu, J.; Xiao, Y.; Sun, R.; Luo, Z.; Cheung, A. M. H.; Zhang, J.; Sun, H.; Zhou, W.; Chen, S.; Guo, X.; Lu, X.; Gao, F.; Min, J.; Yan, H. Fluorinated end group enables high-performance all-polymer solar cells with near-infrared absorption and enhanced device efficiency over 14%. Adv. Energy Mater. 2020, 11, 2003171.
Constructing a new polymer acceptor enabled non-halogenated solvent-processed all-polymer solar cell with an efficiency of 13.8%
Chem. Commun. 2021 57 935 938Zhu, C.; Li, Z.; Zhong, W.; Peng, F.; Zeng, Z.; Ying, L.; Huang, F.; Cao, Y. Constructing a new polymer acceptor enabled non-halogenated solvent-processed all-polymer solar cell with an efficiency of 13.8%. Chem. Commun. 2021, 57, 935−938.
Over 14% efficiency all-polymer solar cells enabled by a low bandgap polymer acceptor with low energy loss and efficient charge separation
Energy Environ. Sci. 2020 13 5017 5027Fan, Q.; An, Q.; Lin, Y.; Xia, Y.; Li, Q.; Zhang, M.; Su, W.; Peng, W.; Zhang, C.; Liu, F.; Hou, L.; Zhu, W.; Yu, D.; Xiao, M.; Moons, E.; Zhang, F.; Anthopoulos, T. D.; Inganäs, O.; Wang, E. Over 14% efficiency all-polymer solar cells enabled by a low bandgap polymer acceptor with low energy loss and efficient charge separation. Energy Environ. Sci. 2020, 13, 5017−5027.
High efficiency (15.8%) all-polymer solar cells enabled by a regioregular narrow bandgap polymer acceptor
J. Am. Chem. Soc 2021 143 2665 2670Fu, H.; Li, Y.; Yu, J.; Wu, Z.; Fan, Q.; Lin, F.; Woo, H. Y.; Gao, F.; Zhu, Z.; Jen, A. K. High efficiency (15.8%) all-polymer solar cells enabled by a regioregular narrow bandgap polymer acceptor. J. Am. Chem. Soc 2021, 143, 2665−2670.
Precisely controlling the position of bromine on the end group enables well-regular polymer acceptors for all-polymer solar cells with efficiencies over 15%
Adv. Mater. 2020 32 e2005942Luo, Z.; Liu, T.; Ma, R.; Xiao, Y.; Zhan, L.; Zhang, G.; Sun, H.; Ni, F.; Chai, G.; Wang, J.; Zhong, C.; Zou, Y.; Guo, X.; Lu, X.; Chen, H.; Yan, H.; Yang, C. Precisely controlling the position of bromine on the end group enables well-regular polymer acceptors for all-polymer solar cells with efficiencies over 15%. Adv. Mater. 2020, 32, e2005942.
A universal fluorinated polymer acceptor enables all-polymer solar cells with >15% efficiency
ACS Energy Lett. 2020 5 3702 3707Peng, F.; An, K.; Zhong, W.; Li, Z.; Ying, L.; Li, N.; Huang, Z.; Zhu, C.; Fan, B.; Huang, F.; Cao, Y. A universal fluorinated polymer acceptor enables all-polymer solar cells with >15% efficiency. ACS Energy Lett. 2020, 5, 3702−3707.
15.4% Efficiency all-polymer solar cells
Sci. China Chem. 2021 64 408 412Zhang, L.; Jia, T.; Pan, L.; Wu, B.; Wang, Z.; Gao, K.; Liu, F.; Duan, C.; Huang, F.; Cao, Y. 15.4% Efficiency all-polymer solar cells. Sci. China Chem. 2021, 64, 408−412.
Progress in poly (3-hexylthiophene) organic solar cells and the influence of its molecular weight on device performance
Adv. Energy Mater. 2018 8 1801001Wadsworth, A.; Hamid, Z.; Bidwell, M.; Ashraf, R. S.; Khan, J. I.; Anjum, D. H.; Cendra, C.; Yan, J.; Rezasoltani, E.; Guilbert, A. A. Y.; Azzouzi, M.; Gasparini, N.; Bannock, J. H.; Baran, D.; Wu, H.; de Mello, J. C.; Brabec, C. J.; Salleo, A.; Nelson, J.; Laquai, F.; McCulloch, I. Progress in poly (3-hexylthiophene) organic solar cells and the influence of its molecular weight on device performance. Adv. Energy Mater. 2018, 8, 1801001.
Effect of polymer molecular weight on J51 based organic solar cells
RSC Adv. 2019 9 14657 14661Zhang, Y.; Xu, X.; Lu, J.; Zhang, S. Effect of polymer molecular weight on J51 based organic solar cells. RSC Adv. 2019, 9, 14657−14661.
Small molecular donor/polymer acceptor type organic solar cells: effect of molecular weight on active layer morphology
Macromolecules 2019 52 8682 8689Zhang, Z.; Wang, T.; Ding, Z.; Miao, J.; Wang, J.; Dou, C.; Meng, B.; Liu, J.; Wang, L. Small molecular donor/polymer acceptor type organic solar cells: effect of molecular weight on active layer morphology. Macromolecules 2019, 52, 8682−8689.
Fine-tuning semiconducting polymer self-aggregation and crystallinity enables optimal morphology and high-performance printed all-polymer solar cells
J. Am. Chem. Soc. 2020 142 392 406Wu, Y.; Schneider, S.; Walter, C.; Chowdhury, A. H.; Bahrami, B.; Wu, H. C.; Qiao, Q.; Toney, M. F.; Bao, Z. Fine-tuning semiconducting polymer self-aggregation and crystallinity enables optimal morphology and high-performance printed all-polymer solar cells. J. Am. Chem. Soc. 2020, 142, 392−406.
Elucidating roles of polymer donor aggregation in all-polymer and non-fullerene small-molecule-polymer solar cells
Chem. Mater. 2020 32 3585 3596Park, J. S.; Choi, N.; Lee, C.; Lee, S.; Ha, J. W.; Hwang, D. H.; Kim, B. J. Elucidating roles of polymer donor aggregation in all-polymer and non-fullerene small-molecule-polymer solar cells. Chem. Mater. 2020, 32, 3585−3596.
Optimisation of processing solvent and molecular weight for the production of green-solvent-processed all-polymer solar cells with a power conversion efficiency over 9%
Energy Environ. Sci. 2017 10 1243 1251Fan, B.; Ying, L.; Wang, Z.; He, B.; Jiang, X. F.; Huang, F.; Cao, Y. Optimisation of processing solvent and molecular weight for the production of green-solvent-processed all-polymer solar cells with a power conversion efficiency over 9%. Energy Environ. Sci. 2017, 10, 1243−1251.
Improving active layer morphology of all-polymer solar cells by solution temperature
Macromolecules 2020 53 3325 3331Wang, N.; Yu, Y.; Zhao, R.; Ding, Z.; Liu, J.; Wang, L. Improving active layer morphology of all-polymer solar cells by solution temperature. Macromolecules 2020, 53, 3325−3331.
Improving active layer morphology of all-polymer solar cells by dissolving the two polymers individually
Macromolecules 2019 52 2402 2410Wang, N.; Long, X.; Ding, Z.; Feng, J.; Lin, B.; Ma, W.; Dou, C.; Liu, J.; Wang, L. Improving active layer morphology of all-polymer solar cells by dissolving the two polymers individually. Macromolecules 2019, 52, 2402−2410.
A synergetic effect of molecular weight and fluorine in all-polymer solar cells with enhanced performance
Adv. Funct. Mater. 2017 27 1603564Chen, S.; An, Y.; Dutta, G. K.; Kim, Y.; Zhang, Z. G.; Li, Y.; Yang, C. A synergetic effect of molecular weight and fluorine in all-polymer solar cells with enhanced performance. Adv. Funct. Mater. 2017, 27, 1603564.
Determining the role of polymer molecular weight for high-performance all-polymer solar cells: its effect on polymer aggregation and phase separation
J. Am. Chem. Soc. 2015 137 2359 2365Kang, H.; Uddin, M. A.; Lee, C.; Kim, K. H.; Nguyen, T. L.; Lee, W.; Li, Y.; Wang, C.; Woo, H. Y.; Kim, B. J. Determining the role of polymer molecular weight for high-performance all-polymer solar cells: its effect on polymer aggregation and phase separation. J. Am. Chem. Soc. 2015, 137, 2359−2365.
, Elucidating the impact of molecular weight on morphology, charge transport, photophysics and performance of all-polymer solar cells
J. Mater. Chem. A 2020 8 21070 21083Tran, D. K.; Robitaille, A.; Hai, I. J.; Ding, X.; Kuzuhara, D.; Koganezawa, T.; Chiu, Y. C.; Leclerc, M.; Jenekhe, S. A. , Elucidating the impact of molecular weight on morphology, charge transport, photophysics and performance of all-polymer solar cells. J. Mater. Chem. A 2020, 8, 21070−21083.
Morphology optimization via molecular weight tuning of donor polymer enables all-polymer solar cells with simultaneously improved performance and stability
Nano Energy 2019 64 103931Li, Z.; Zhong, W.; Ying, L.; Liu, F.; Li, N.; Huang, F.; Cao, Y. Morphology optimization via molecular weight tuning of donor polymer enables all-polymer solar cells with simultaneously improved performance and stability. Nano Energy 2019, 64, 103931.
Photoactive blend morphology engineering through systematically tuning aggregation in all-polymer solar cells
Adv. Energy Mater. 2018 8 1702173Wang, G.; Eastham, N. D.; Aldrich, T. J.; Ma, B.; Manley, E. F.; Chen, Z.; Chen, L. X.; de la Cruz, M. O.; Chang, R. P. H.; Melkonyan, F. S.; Facchetti, A.; Marks, T. J. Photoactive blend morphology engineering through systematically tuning aggregation in all-polymer solar cells. Adv. Energy Mater. 2018, 8, 1702173.
All-polymer solar cell performance optimized via systematic molecular weight tuning of both donor and acceptor polymers
J. Am. Chem. Soc. 2016 138 1240 1251Zhou, N.; Dudnik, A. S.; Li, T. I.; Manley, E. F.; Aldrich, T. J.; Guo, P.; Liao, H. C.; Chen, Z.; Chen, L. X.; Chang, R. P.; Facchetti, A.; Olvera de la Cruz, M.; Marks, T. J. All-polymer solar cell performance optimized via systematic molecular weight tuning of both donor and acceptor polymers. J. Am. Chem. Soc. 2016, 138, 1240−1251.
Organoboron polymer for 10% efficiency all-polymer solar cells
Chem. Mater. 2020 32 1308 1314Zhao, R.; Wang, N.; Yu, Y.; Liu, J. Organoboron polymer for 10% efficiency all-polymer solar cells. Chem. Mater. 2020, 32, 1308−1314.
Organic solar cells based on small molecule donors and polymer acceptors operating at 150 °C
J. Mater. Chem. A 2020 8 10983 10988Miao, J.; Meng, B.; Ding, Z.; Liu, J.; Wang, L. Organic solar cells based on small molecule donors and polymer acceptors operating at 150 °C. J. Mater. Chem. A 2020, 8, 10983−10988.
Amorphous polymer acceptor containing B ← N units matches various polymer donors for all-polymer solar cells
Macromolecules 2019 52 7081 7088Zhao, R.; Lin, B.; Feng, J.; Dou, C.; Ding, Z.; Ma, W.; Liu, J.; Wang, L. Amorphous polymer acceptor containing B ← N units matches various polymer donors for all-polymer solar cells. Macromolecules 2019, 52, 7081−7088.
High-performance all-polymer solar cells: synthesis of polymer acceptor by a random ternary copolymerization strategy
Angew. Chem. Int. Ed. 2020 59 15181 15185Du, J.; Hu, K.; Meng, L.; Angunawela, I.; Zhang, J.; Qin, S.; Liebman-Pelaez, A.; Zhu, C.; Zhang, Z.; Ade, H.; Li, Y. High-performance all-polymer solar cells: synthesis of polymer acceptor by a random ternary copolymerization strategy. Angew. Chem. Int. Ed. 2020, 59, 15181−15185.
Random copolymerization realized high efficient polymer solar cells with a record fill factor near 80%
Nano Energy 2019 61 228 235Xie, Q.; Liao, X.; Chen, L.; Zhang, M.; Gao, K.; Huang, B.; Xu, H.; Liu, F.; Jen, A. K. Y.; Chen, Y. Random copolymerization realized high efficient polymer solar cells with a record fill factor near 80%. Nano Energy 2019, 61, 228−235.
A simple strategy to the side chain functionalization on the quinoxaline unit for efficient polymer solar cells
Chem. Commun. 2016 52 6881 6884Yuan, J.; Qiu, L.; Zhang, Z.; Li, Y.; He, Y.; Jiang, L.; Zou, Y. A simple strategy to the side chain functionalization on the quinoxaline unit for efficient polymer solar cells. Chem. Commun. 2016, 52, 6881−6884.
Optimized fibril network morphology by precise side-chain engineering to achieve high-performance bulk-heterojunction organic solar cells
Adv. Mater. 2018 30 e1707353Liu, T.; Huo, L.; Chandrabose, S.; Chen, K.; Han, G.; Qi, F.; Meng, X.; Xie, D.; Ma, W.; Yi, Y.; Hodgkiss, J. M.; Liu, F.; Wang, J.; Yang, C.; Sun, Y. Optimized fibril network morphology by precise side-chain engineering to achieve high-performance bulk-heterojunction organic solar cells. Adv. Mater. 2018, 30, e1707353.
Optimal bulk-heterojunction morphology enabled by fibril network strategy for high-performance organic solar cells
Sci. China Chem. 2019 62 662 668Xia, T.; Cai, Y.; Fu, H.; Sun, Y. Optimal bulk-heterojunction morphology enabled by fibril network strategy for high-performance organic solar cells. Sci. China Chem. 2019, 62, 662−668.
An optimized fibril network morphology enables high-efficiency and ambient-stable polymer solar cells
Adv. Sci. 2020 7 2001986Song, J.; Ye, L.; Li, C.; Xu, J.; Chandrabose, S.; Weng, K.; Cai, Y.; Xie, Y.; O'Reilly, P.; Chen, K.; Zhou, J.; Zhou, Y.; Hodgkiss, J. M.; Liu, F.; Sun, Y. An optimized fibril network morphology enables high-efficiency and ambient-stable polymer solar cells. Adv. Sci. 2020, 7, 2001986.
Fibril network strategy enables high-performance semitransparent organic solar cells
Adv. Funct. Mater. 2020 30 2002181Xie, Y.; Cai, Y.; Zhu, L.; Xia, R.; Ye, L.; Feng, X.; Yip, H. L.; Liu, F.; Lu, G.; Tan, S.; Sun, Y. Fibril network strategy enables high-performance semitransparent organic solar cells. Adv. Funct. Mater. 2020, 30, 2002181.
Device physics of polymer:fullerene bulk heterojunction solar cells
Adv. Mater. 2007 19 1551 1566Blom, P. W. M.; Mihailetchi, V. D.; Koster, L. J. A.; Markov, D. E. Device physics of polymer:fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551−1566.
Tuning the dipole moments of nonfullerene acceptors with an asymmetric terminal strategy for highly efficient organic solar cells
J. Mater. Chem. A 2019 7 8889 8896Li, M.; Zhou, Y.; Zhang, J.; Song, J.; Bo, Z. Tuning the dipole moments of nonfullerene acceptors with an asymmetric terminal strategy for highly efficient organic solar cells. J. Mater. Chem. A 2019, 7, 8889−8896.