INTRODUCTION
AIE materials with long-wave emission are attracting people’s extensive attention and interest in many fields due to their various advantages such as less harm, higher sensitivity, and deeper imaging depth, etc.[1−7] Heterocyclic compounds of five- or six-membered rings have been extensively studied since their discovery. The heteroatoms have also evolved from the original O, N and S atoms to a variety of metallic and non-metallic atoms, and the application of these heterocyclic compounds extends to bioengineering, bioimaging, dye chemistry and many other fields. A large proportion of these heterocyclic compounds can emit fluorescence with long-wave emission, such as phenothiazine, benzothiadiazole and organoboron compounds, etc., so they are widely used as organic fluorescent materials in organic optoelectronic devices, fluorescence chemical analysis, biomedical tracer and so on.[8−15] For example, benzo-2,1,3-thiadiazole moieties can also strongly shift the emission wavelength from blue to red with adjusting the fluorescence characteristics of aggregation-enhanced emission.[16] PIPBT-TPE and PITBT-TPE with N and S atoms heterocyclic rings can emit green fluorescence at 560 nm and red fluorescence at 645 nm, and they were applied for real-time fluorescence in vivo blood visualization due to the excellent biocompatibility and prospective candidates for living cells and tissue imaging.[17] BTD-TPA and BTD-NPA red luminogens with benzothiadiazole and arylamino moieties showed aggregation-induced emission (AIE) features with about 600 nm emission wavelength, which could be used as important biomolecules for sensitive detection of dopamine (DA) with high selectivity.[18] Another pH-sensitive fluorescent probe of imidazole-fused benzothiadiazole was synthesized with the excellent fluorescence properties and lysosomal targeting ability, and it has been successfully used to monitor the pH variety in lysosomes which were induced by Baf-A1 or chloroquine.[19] Similarly, a new class of phenanthroimidazole-based organic boron compounds have been synthesized. Their solution-state fluorescence quantum yields range from 0.07 to 0.88, and their solid-state quantum yields are moderate, which are used in organic light-emitting diodes and fluorescent probes.[20]
The phenothiazine with the heteroatomic six-membered ring has a bifacial angle of 153°, presenting a twisted butterfly-like structure, which makes itself both rigid and flexible. At the same time, phenothiazine has a relatively strong electron-donating ability, and its introduction into the molecular structure can reduce the energy level difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), making the emission wavelength of the molecule increase, and can be used as an electron donor (D) to construct D-A fluorescent molecules with red light emission.[21−23] For example, a series of novel phenothiazine chalcone derivatives were constructed with the D-π-A structure and low band gap energy, and they had obvious red emission, aggregation-induced emission enhancement (AEE) characteristics and reversible "turn-on" mechanofluorochromism behavior, which made them potential candidates for the manufacture of color-changing switches and piezoelectric smart optoelectronic devices.[24] Moreover, a series of new derivatives of PEGylated phenothiazines were produced by grafting PEGMA to phenothiazine, which could assemble to form submicrometric aggregates and induce fluorescence emission due to the good solubility and hydrolytic stability. These derivatives not only retained the potential for the preparation of biological materials, but also could be applied in the field of optoelectronics.[25]
Chirality is a ubiquitous feature in nature and organisms, involving a wide range from small molecules to large molecules, and nature seems to have a selective preference for the chirality of specific molecules. Almost all the amino acids that make up the lives of the earth are levorotatory, while the sugar units and DNA in sugars and nucleic acids are dextrorotatory. The ingenious design contains philosophy: different enantiomers of the same molecule often have very different effects on the organism, which makes the feature of chirality be vital in life science and biomedicine. With the vigorous development in the areas of life sciences, medicine, and chemistry science, researches on understanding and exploration of opponent molecules are also deepening with extensive achievements.[26−30] The first Nobel prize of chemistry in the 21st century was awarded to chirality catalysis. In recent years, due to the superior performance of the polymer itself, chirality research has gradually extended to chiral polymers, which have prospective applications in the areas of chiral recognition, racemic separation, asymmetric synthesis, chiral catalysis, liquid crystal, drug synthesis, etc.[31−36]
In consideration of the excellent fluorescence and long-wave emission property of phenothiazine in the aggregated state, in this study, a novel polymerizable fluorescent (R)-PVHMA dye with AEE and chirality features was acquired through Suzuki coupling reaction and enzymatic transesterification using phenothiazine as the basic skeleton. The obtained (R)-PVHMA monomer has a specific rotation 
EXPERIMENTAL
Materials and Characterization
1-Bromooctadecane (Aladdin, 97%), potassium tert-butanol (Aladdin, 95%), phosphoryl chloride (Xiya Reagent Co., Ltd., 99%), 2,2,2-trifluoroethyl methacrylate (TFEMA) (Aladdin, 98%), 6-chloro-2-hexanone (Aladdin, 97%), tetrabutylammoniumhydroxide solution (TBAH) (Aladdin, ~25% in methanol, ~0.8 mol/L), phenothiazine (Aladdin, 98%), sodium iodide, 1,2-dichloroethane (Aladdin, 99.8%), 4-hydroxyphenylacetonitrile (Aladdin, 98%), 2,2-azobisisobutyronitrile (AIBN) (Aladdin, 99%), Novozym 435, potassium carbonate, triethylamine (TEA) and sodium bicarbonate were purchased directly for use. Poly(ethylene glycol) monomethacrylate (PEGMA) (Aladdin, Mn=475, AR) was purified through an aluminum oxide column before use. The solvents such as n-hexane, methylene chloride, etc. used directly in the experiment were analytical reagent. 6-Chloro-2-hexanol (CHOL),10-octadecyl-10H-phenothiazine-3-carbaldehyde (OPC) and chain transfer agent (CTA) of 4-cyano-4-(ethylthiocarbonothioylthio) pentanoic acid were self-made in the laboratory according to the reported references.[37−40]
The chemical structure of the target products was determined by hydrogen spectroscopy of nuclear magnetic resonance (1H-NMR) and Fourier transform infrared (FTIR) spectroscopy. The 1H-NMR spectroscopy was measured on a JEOL JNM-ECA 400 (400 MHz) spectrometer with chloroform (CDCl3) as the solvent and tetramethylsilane (TMS) as the reference. Infrared spectra were acquired on a PerkinElmer TWO spectrometer. The molecular weight (Mn, g/mol) and the polydispersity index (PDI) were obtained from a gel permeation chromatograph (GPC) model of a Waters 1515 equipment using tetrahydrofuran (THF) as mobile phase at 1.0 mL/min flow rate. Transmission electron microscopy (TEM) images of copolymer nanoparticles were obtained on a transmission electron microscope model of JEM-1200EX at an operating voltage of 100 kV. The preparation process of the test sample was as follows: 10 mg of the target copolymers were added into 4 mL of deionized water and dissolved completely, and then added a drop of mixed solution onto the carbon-coated copper grids and let it stand until dry. Ultraviolet-visible spectra (UV-Vis) were recorded on a Perkin-Elmer LAMBDA 35 spectrophotometer. The fluorescence curves were acquired on a Hitachi F-2700 spectrophotometer. The specific rotation of the (R)-PVHMA monomer and PEG-PVH was measured on an SGW-3 autopolarizer in acetone (concentration=10 mg/mL) at 25 °C.
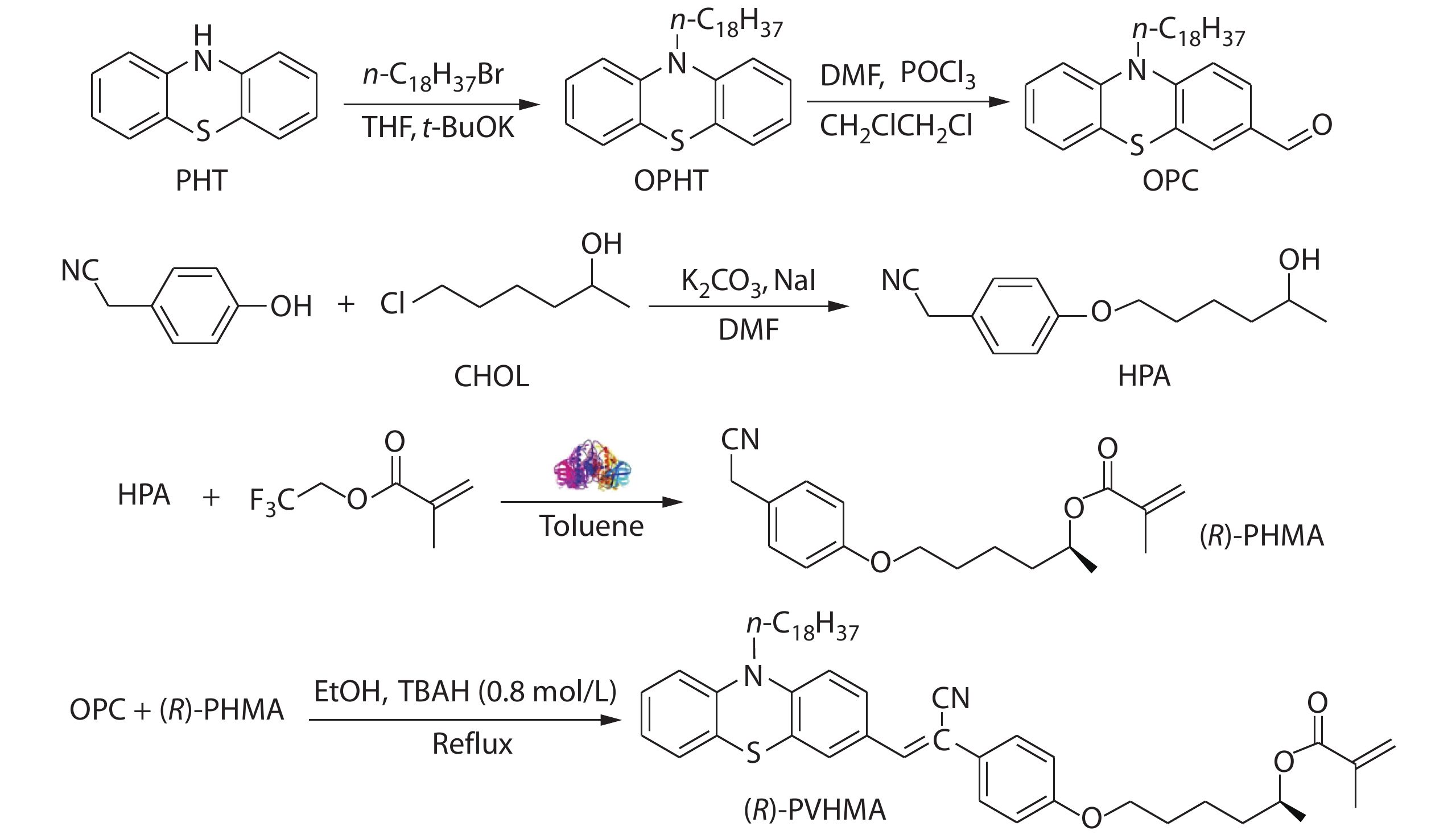
Synthesis of 2-(4-((5-Hydroxyhexyl)oxy)phenyl)- acetonitrile (HPA)
4-Hydroxyphenylacetonitrile (1.13 g, 8.5 mmol), CHOL (1.50 g, 11.0 mmol), potassium carbonate (2.93 g, 21.25 mmol), sodium iodide (140 mg), DMF (35.0 mL) and a magnetic stir bar were added into a Schlenk tube, and then this tube was sealed, frozen in liquid nitrogen and deoxidized for five times of vacuum-nitrogen cycles. The Schlenk tube was then heated and stirred at 100 °C for 24 h in an oil bath. Afterwards, the pH was neutralized to 4−5 with 3 mol/L of hydrochloric acid. An appropriate amount of distilled water was put into the Schlenk tube, and then the organic phase was extracted for three times using ethyl acetate and dried with anhydrous MgSO4. The reaction mixture was further filtered to remove MgSO4, and the crude product was acquired through removing the solvent on a rotary evaporator. The product was further purified on a silica column using petroleum ether/ethyl acetate (V/V=1/1) as mobile phase, and the pure product HPA was obtained after completely drying under vacuum (1.61 g, 81%). 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.23−1.24 (d, J=4.0 Hz, 3H; CH3), 1.53−1.56 (m, 4H; ―CH2―), 1.60−1.64 (m, 1H; ―OH), 1.81−1.85 (m, 2H; ―CH2―), 3.70 (s, 2H; ―CH2CN), 3.84−3.88 (m, 1H; ―CH), 3.97−4.00 (t, J=6.0 Hz, 2H; ―CH2Cl), 6.89−6.91 (d, J=6.0 Hz, 2H; ArH), 7.23−7.25 (d, J=8.0 Hz, 2H; ArH).
Synthesis of (R)-6-(4-(Cyanomethyl)phenoxy)hexan-2-yl Methacrylate ((R)-PHMA)
Novozym435 (294 mg) and a magnetic stir bar were added into a Schlenk tube, and then this tube was sealed and deoxidized for five times by vacuum-nitrogen cycling. HPA (1.63 g, 7.0 mmol), TFEMA (0.71 g, 4.2 mmol) and TEA (0.35 g, 3.5 mmol) were completely dissolved in toluene (20.0 mL), followed by injection of the mixture into the Schlenk tube, which was put into an oil bath at 55 °C and stirred for 24 h. Subsequently, Novozym435 catalytic agent was eliminated through centrifugation, and then the solvent of the crude product was eliminated through a rotary evaporator. Finally, the product was purified through a silica column taking petroleum ether/ethyl acetate (V/V=2/1) as eluent, and the product (R)-PHMA was obtained by drying under vacuum (0.66 g, 63%). 1H-NMR (400 MHz, CDCl3, δ, ppm): 1.28−1.30 (d, J=8.0 Hz, 3H; ―CH3), 1.50−1.70 (m, 4H; ―CH2―), 1.77−1.84 (m, 2H; ―CH2―), 1.96 (s, 3H; ―CH3), 3.70 (s, 2H; ―CH2CN), 3.95−3.98 (t, J=6.0 Hz, 2H; ―CH2Cl), 5.00−5.04 (m, 1H; ―CH), 5.56 (s, 1H; ―CH), 6.10 (s, 1H; ―CH), 6.89−6.90 (d, J=4.0 Hz, 2H; ArH), 7.23−7.25 (d, J=8.0 Hz, 2H; ArH).
Synthesis of (R)-6-(4-(1-Cyano-2-(10-octadecane-10H-phenothiazin-3-yl)vinyl)phenoxy)hexan-2-yl methacrylate ((R)-PVHMA)
OPC (1.16 g, 2.42 mmol), (R)-PHMA (0.66 g, 2.2 mmol), TBAH (5−6 drops), ethanol (20.0 mL) and a magnetic stir bar were put into a Schlenk tube, and then this tube was stirred magnetically for 0.5 h at 30 °C to make the mixture completely dissolve. The Schlenk tube was then heated and stirred for 5 h at 78 °C. At the end of the reaction, the product was obtained by removing the solvent on a rotary evaporator, which was further purified through a silica column taking dichloromethane/n-hexane (V/V=2:1) as eluent, and the pure product (R)-PVHMA was dried under vacuum (1.00 g, 60%). 1H-NMR (400 MHz, CDCl3, δ, ppm): 0.88−0.92 (t, J=8.0 Hz, 3H, ―CH3), 1.20−1.34 (m, 29H, ―C13H26―, ―CH3), 1.42−1.48 (m, 2H, ―CH2―), 1.52−1.67 (m, 6H, ―CH2―), 1.80−1.87 (m, 4H, ―CH2―), 1.97 (s, 3H, ―CH3), 3.90 (s, 2H, ―CH2―), 4.00−4.03 (t, J=6.0 Hz, 2H, ―CH2Cl), 5.00−5.04 (m, 1H, ―CH), 5.56 (s, 1H, ―CH), 6.11 (s, 1H, ―CH), 6.88−6.89 (d, J=4.0 Hz, 2H, ArH), 6.93−6.95 (d, J=8.0 Hz, 3H, ArH), 7.14 (m, 2H, ArH), 7.32−7.34 (d, J=4.0 Hz, 1H, ArH), 7.55 (s, 1H, Ar-CH), 7.56−7.58 (d, J=8.0 Hz, 2H, ArH), 7.80−7.82 (d, J=8.0 Hz, 1H, ArH). 13C-NMR (400 MHz, CDCl3, Fig. S1 in the electronic supplementary information, ESI): 14.13, 17.93, 19.97, 21.31, 22.01, 22.70, 26.94, 29.67, 31.89, 35.75, 67.94, 70.86, 115.00, 124.73, 127.37, 137.11. The molecular weight by TOFMS=762.5 (Fig. S2 in ESI).
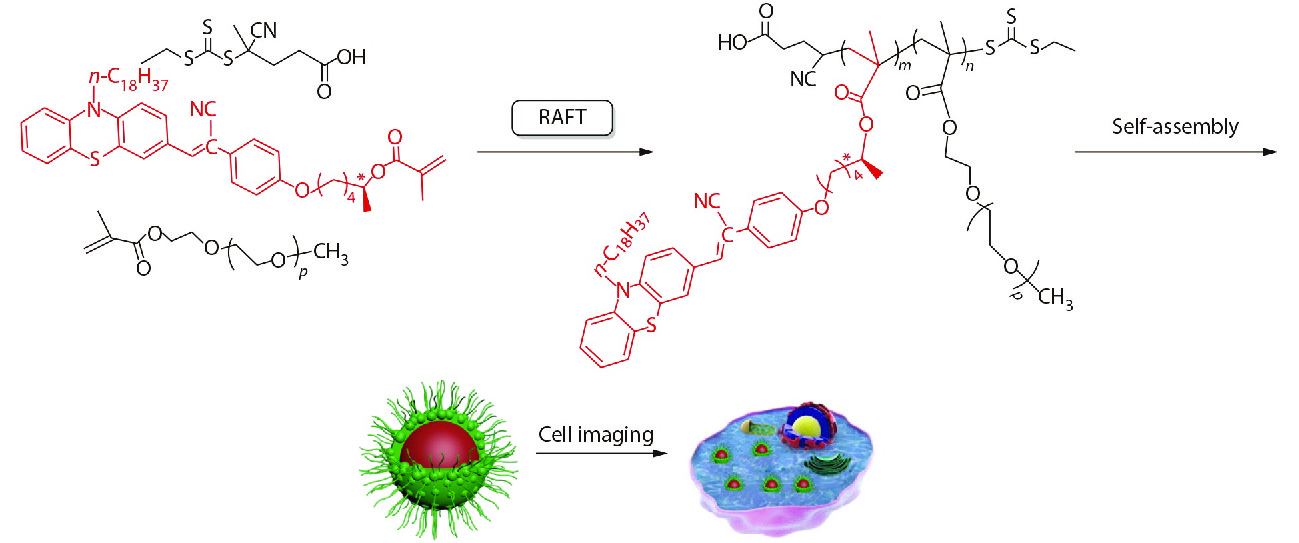
Synthesis of PEG-PVH Amphiphilic Copolymers
(R)-PVHMA (104.3 mg, 0.137 mmol), AIBN (1.0 mg, 0.006 mmol), PEGMA (237 mg, 0.499 mmol), CTA (3.1 mg, 0.012 mmol), toluene (2.0 mL) and a magnetic stir bar were placed into a Schlenk tube, subsequently, this tube was sealed, frozen in liquid N2 and then deoxygenated by vacuum-nitrogen cycles for five times. The Schlenk tube was then heated and stirred at 70 °C for 36 h. After the reaction was completed, the mixture was continually dialyzed with acetone for three times for 12 h each. The crude product was obtained by removing the organic solvent on a rotary evaporator, and then purified by multiple cycles of tetrahydrofuran dissolution and petroleum ether precipitation, and the resulted PEG-PVH1 copolymers were dried under vacuum with the yield of 0.26 g. Another PEG-PVH2 copolymers were fabricated according to the same method as described above, where the molar ratio of (R)-PHMA was adjusted to 29.6%, yield: 0.27 g.
RESULTS AND DISCUSSION
Characterization and Features of PEG-PVH FONs
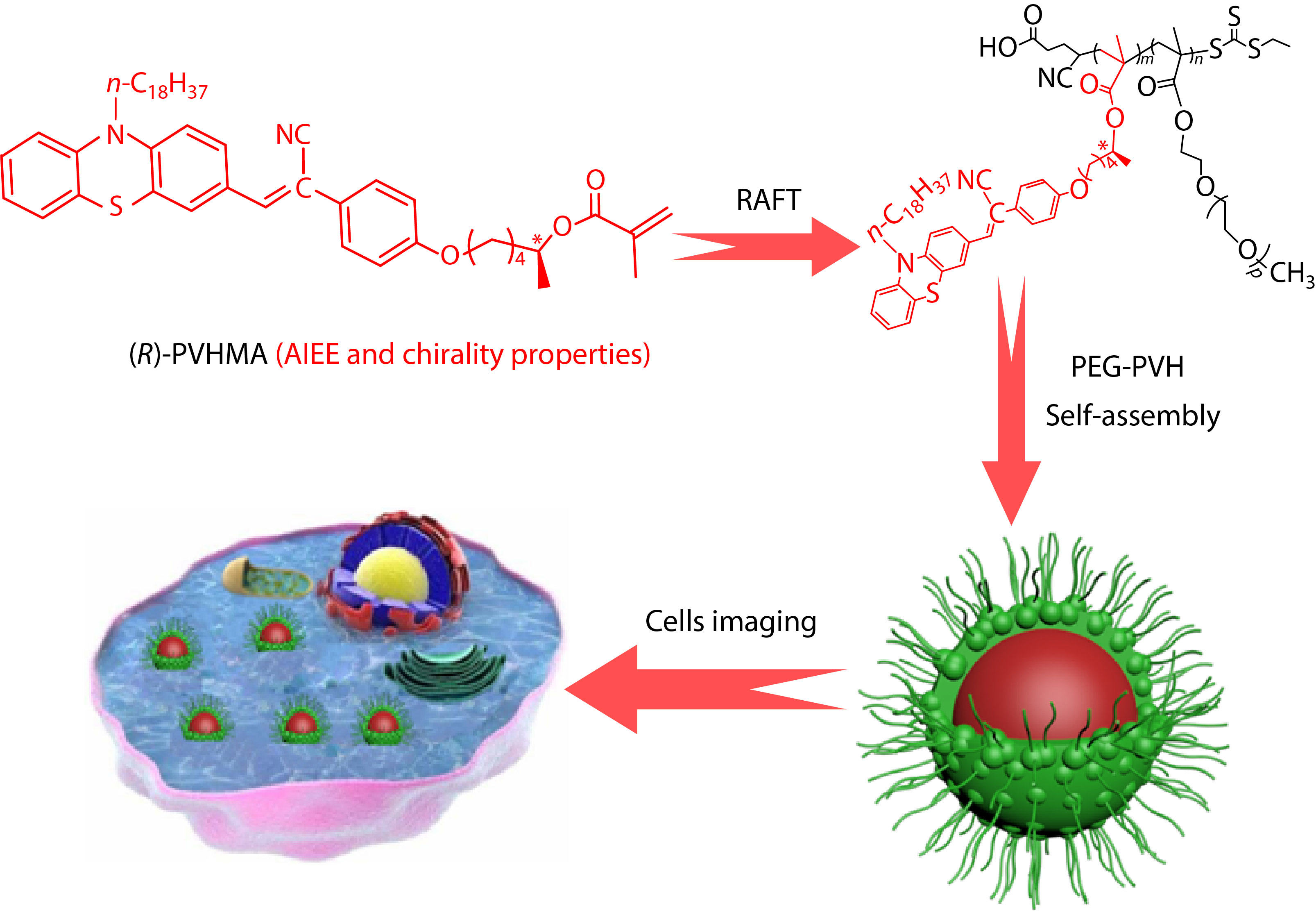
Scheme 1 shows the synthetic route to the fluorescence monomer (R)-PVHMA and this process can be divided into three parts. Firstly, the nucleophilic substitution reaction of phenothiazine (PHT) and 1-bromooctadecane was used to obtain the intermediate product 10-octadecanephenothiazine (OPHT), which was followed by a Vilsmeier-Haack reaction with DMF catalyzed by phosphoryl chloride to obtain the OPC. Secondly, HPA was obtained by the nucleophilic substitution reaction of 4-hydroxyphenylacetonitrile and CHOL, and it would continually perform the transesterification reaction with TEFMA catalyzed by Novozym435 to obtain chiral molecule (R)-PHMA with R conformation through the efficient chiral resolution of racemic secondary alcohol. Thirdly, the aldehyde group in the OPC structure will undergo a Knoevenagel condensation reaction with the active methylene of (R)-PHMA and obtain the chiral fluorescence monomer (R)-PVHMA. Subsequently, the RAFT polymerization of (R)-PVHMA with PEGMA was used to prepare the corresponding amphiphilic PEG-PVH copolymers with chirality property, which tended to self-assemble into nanoparticles due to their amphiphilicity and could be easily absorbed by cells, as exhibited in Scheme 2.
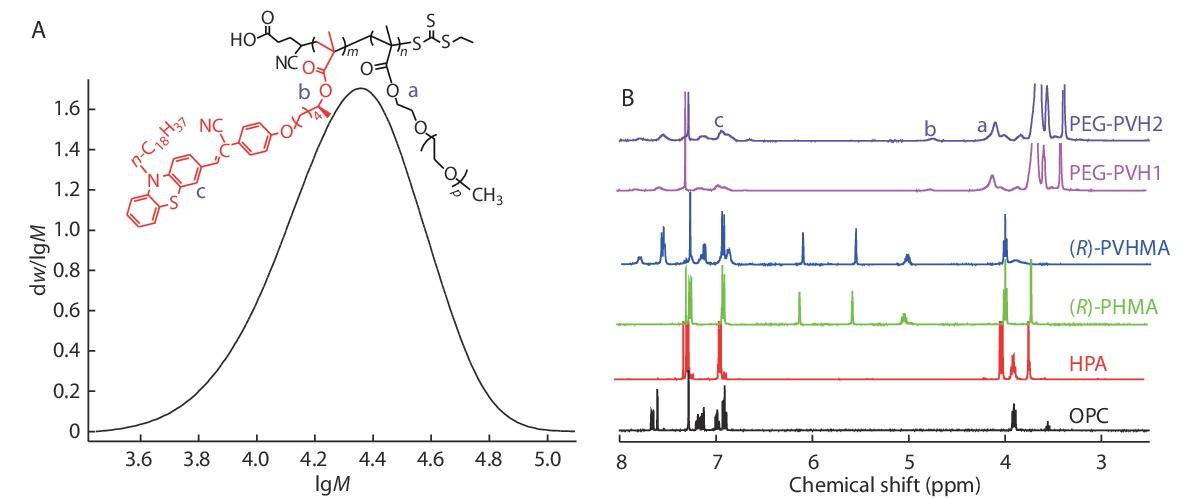
The GPC data for the target PEG-PVH1 copolymers was exhibited in Fig. 1(A), the Mn value was about 2.2×104, and their PDI was about 1.30. The structures of the individual target substances OPC, HPA, (R)-PHMA, (R)-PVHMA and PEG-PVH were investigated by 1H-NMR as exhibited in Fig. 1(B). A simple substitution reaction of phenothiazine and 1-bromooctadecane was used to construct the intermediate product OPHT, which was formylated by DMF to produce OPC through the catalysis of phosphoryl chloride, and the peak assigned to the characteristic proton of the introduced formyl group appeared at 9.80 ppm. At the same time, the triplet peaks at 3.90 ppm originated from the methylene ―CH2― adjacent to the N atom, indicating that the introduced octadecyl chain was not affected by the formylation reaction. Hydrocarbylation reaction of 4-hydroxyphenylacetonitrile and CHOL formed the corresponding aromatic ether HPA. In the 1H-NMR spectrum of HPA, the peaks assigned to the proton of ―CH2=CN appeared at 3.70 ppm, and the characteristic proton peak of the methylene ―CH2― linking to the O atom appeared at 3.98 ppm. More importantly, the characteristic peak of the H atom connecting to the chiral C atom appeared at 3.85 ppm. Due to the high enantioselectivity of Novozym435 to racemic secondary alcohol HPA, R configuration HPA had an absolute advantage in the transesterification reaction with TEFMA, and thus the corresponding R configuration chiral intermediate (R)-PHMA was obtained. As compared with HPA, the 1H-NMR spectrum of (R)-PHMA not only retained the typical peaks at 3.70 and 3.98 ppm, but also presented characteristic single peaks at 5.56 and 6.10 ppm deriving from ―C=CH2. Otherwise, the resonant absorption peak of the H atom attached to the chiral C atom transferred to the lower field and appeared at 5.02 ppm from 3.85 ppm due to the successful introduction of the electron-withdrawing methacrylate group, which would result in a decrease of the electrons cloud density and an increase of chemical shift. The chiral fluorescent monomer (R)-PVHMA was constructed by the Knoevenagel condensation reaction of aldehyde group and active methylene group, which connected OPC and (R)-PHMA through a C=C bond. For the 1H-NMR spectrum of (R)-PVHMA, the peak at 3.70 ppm assigned to the ―CH2―CN disappeared completely, while the characteristic proton peaks at 3.98, 5.02, 5.56 and 6.10 ppm were unchanged, in addition, the peak of ―CH2― linking to the N atom still appeared at 3.90 ppm, and these results implied the successful Knoevenagel condensation reaction between OPC and (R)-PHMA. Basing on RAFT polymerization of (R)-PVHMA and PEGMA, the ―C=CH2 double bonds in the structure were broken and the characteristic proton peaks at 5.56 and 6.10 ppm assigned to ―C=CH2 completely disappeared. At the same time, the electron density of the H atom bonding to the chiral C atom increased slightly, and its characteristic peak shifted slightly towards the high field, appearing at 4.75 ppm. The peaks of methylene ―CH2― linking to the ester group in the PEGMA were presented at 4.10 ppm, and the typical peaks of aromatic rings in (R)-PVHMA at 6.90−7.80 ppm. The disappearance of the peaks at 5.56 and 6.10 ppm implied that (R)-PVHMA and PEGMA polymerized successfully into the PEG-PVH copolymers through RAFT polymerization. A rough estimation demonstrated that the molar ratio of (R)-PVHMA in the PEG-PVH1 and PEG-PVH2 copolymers were about 18.1% and 25.7% according to the peaks area ratio of 4.10 ppm and 6.90−7.80 ppm, respectively.
The (R)-PVHMA and PEGMA fragments were respectively hydrophobic and hydrophilic, so the obtained copolymers PEG-PVH were amphiphilic, making them easily disperse in aqueous solution and self-assemble into nanoparticles. PEGMA fragments were affinity with water and stretched into water, while hydrophobic (R)-PVHMA fragments were estranged from water and aggregated together which induced fluorescence emission. The stretching PEGMA fragments were considered as a shell and the aggregating (R)-PVHMA fragment as a core, so the copolymers were dispersed into aqueous solution as a fluorescent organic nanoparticle (FONs) with a core-shell structure. The TEM image confirmed this result, as shown in Fig. 2(a), where PEG-PVH1 FONs were spherical particles with about 100 nm in diameter, which also indirectly confirmed the successful combination of the two monomers (R)-PVHMA and PEGMA into the copolymers PEG-PVH.
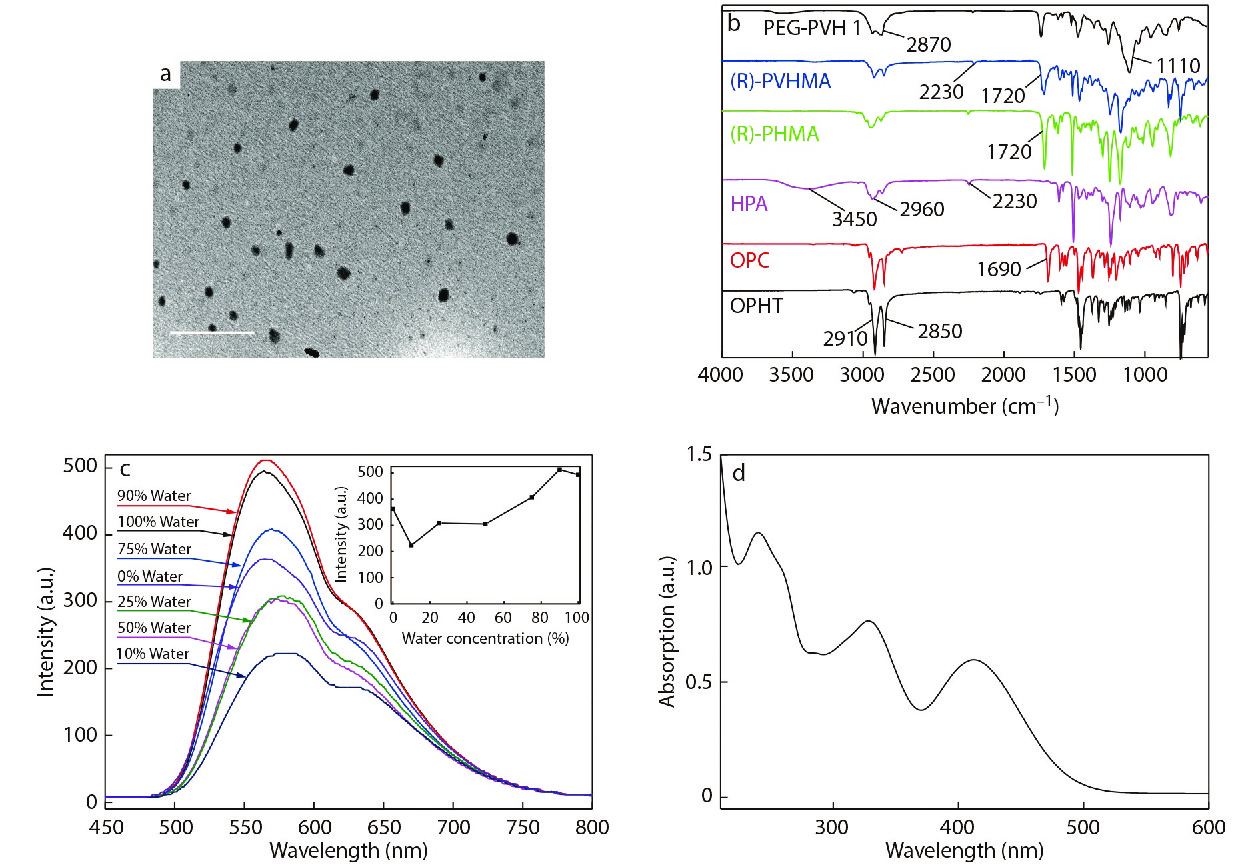
In order to further research the structure of the individual target products OPHT, OPC, HPA, (R)-PHMA, (R)-PVHMA and PEG-PVH, their FTIR spectra were analyzed and the results were shown in Fig. 2(b). The introduction of octadecyl group into phenothiazine was performed through a simple nucleophilic substitution reaction, and the FTIR spectrum reflected the successful preparation of OPHT because the typical stretching bands of alkanes obviously were presented at 2850 and 2910 cm−1. In the FTIR spectrum of OPC, a vibration band of the introduced C=O group evidently appeared at 1690 cm−1, and at the same time, the octadecyl structure remained unchanged. In the curve of HPA, the absorption bands at 2230, 3450 and 2960 cm−1 were assigned to the vibration of cyano C≡N, hydroxy O―H and alkyl chains, respectively, which implied that the hydrocarbylation reaction of 4-hydroxyphenylacetonitrile and CHOL was successful. As compared with HPA, the band at 3450 cm−1 completely disappeared in the curve of (R)-PHMA, while a typical vibration peak of C=O group appeared at 1720 cm−1, which indicated that the alcohol hydroxyl groups in the HPA structure were completely depleted and the transesterification reaction between HPA and TFEMA was successful. Moreover, the high enantioselectivity of Novozym435 to the racemic HPA was utilized to realize the construction of intermediate (R)-PHMA with R configuration. Compared with (R)-PHMA, the FTIR spectrum of (R)-PVHMA also contained absorption bands at 1720, 2230 and 2960 cm−1, indicating that (R)-PVHMA structure contained (R)-PHMA fragments. The difference mainly concentrated on the range of 1580−1500 cm−1 and 800−750 cm−1, which reflected the stretching vibration and out-of-plane deformation vibration of the conjugated aromatic ring skeleton, respectively. The stretching vibrations of C=O, ―CH2― and C―O brought about three distinct absorption bands of 1720, 2870 and 1100 cm−1 respectively, in the spectrum of the PEG-PVH1 copolymers. The structure of the obtained copolymers included typical characteristics of (R)-PVHMA and PEGMA, implying that (R)-PVHMA and PEGMA were copolymerized successfully into the PEG-PVH1 copolymers.
The fluorescence curves of PEG-PVH1 dispersed in THF/H2O solvents with various volume ratios are exhibited in Fig. 2(c). In THF good solvent, the copolymers PEG-PVH1 could emit fluorescence. The reason was that (R)-PVHMA had a relatively strong electron-donating ability and formed a D-A structure with a strong electron-withdrawing phenylacetonitrile group, so the intramolecular charge transfer (ICT) effect would emit fluorescence.[41−43] The fluorescence intensity was slightly reduced when the volume of water was about 10% in the mixed solution, which is caused by a twisted intramolecular charge transfer (TICT) with solvent polarity.[44] With the volume fraction of H2O in the mixed solvent continually increasing, the fluorescence intensity gradually increased. In water, the fluorescence intensity showed a very remarkable improvement and the maximum fluorescence wavelength of PEG-PVH1 FONs was about 580 nm with a shoulder peak at 630 nm. The reason was that the copolymers PEG-PVH1 gradually aggregated along with the increase of H2O fraction in the solution, which hindered the intramolecular motion and activated the restriction of intramolecular motion (RIM), indicating the obvious aggregation-induced emission enhancement (AEE) phenomenon. Amphiphilic copolymers PEG-PVH exhibited self-assemble properties in water, forming corresponding PEG-PVH FONs, whose UV-Vis spectrum was exhibited in Fig. 2(d), where there were three peaks at 240, 330 and 410 nm, respectively. The π→π* electron transition of polycyclic aromatic rings brought the absorption peak at 240 nm, at the same time, due to the presence of hetero atoms O and N in the molecular structure of the copolymers, the n→π* electron transition of their lone electrons resulted in an absorption peak at 330 nm. As mentioned earlier, (R)-PVHMA had a relatively strong electron-donating capacity and there was an intramolecular charge transfer between electron-withdrawing groups such as cyano, carbonyl groups and phenothiazine, which leaded to an absorption peak at 410 nm.[23]
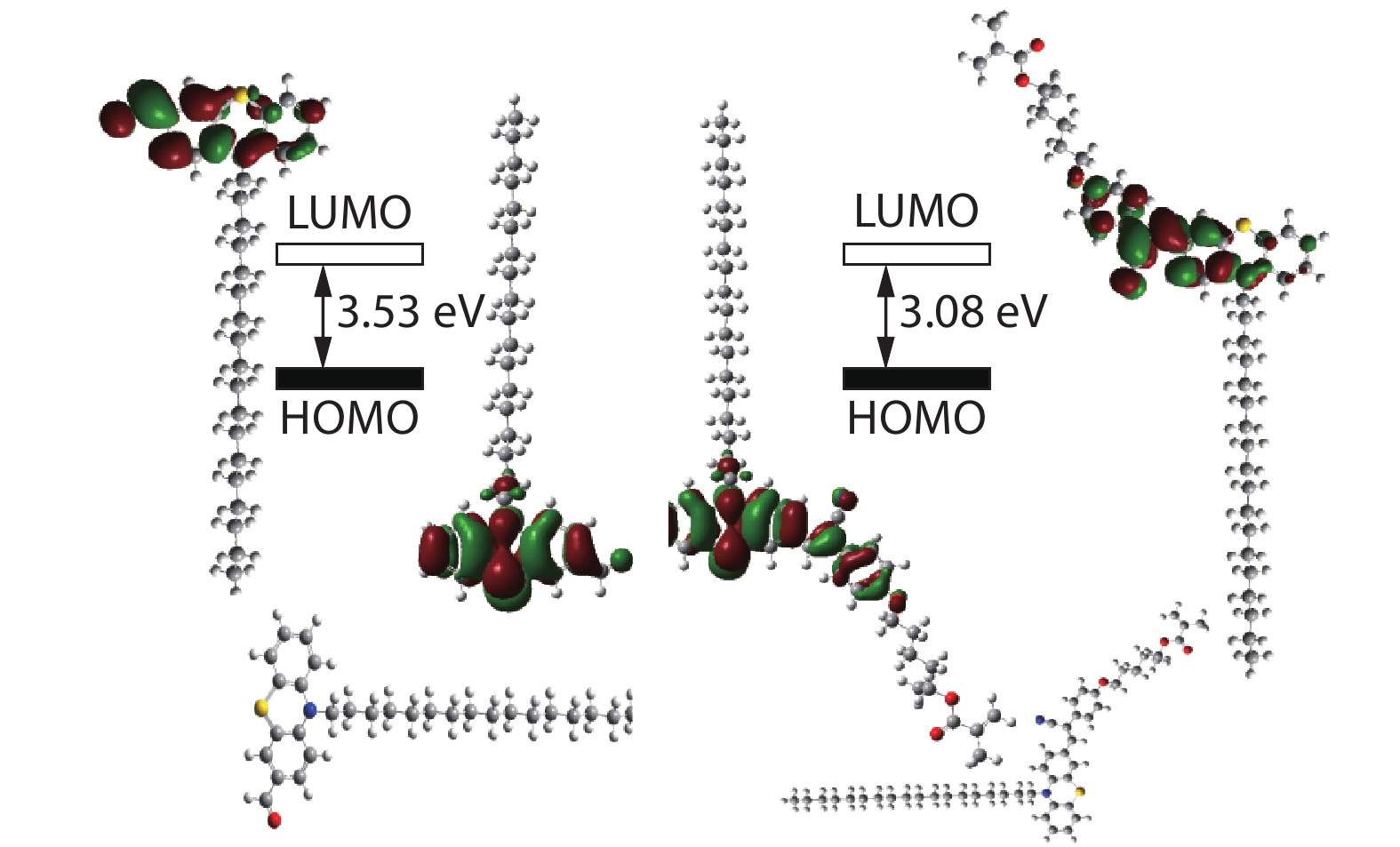
Moreover, as shown in Fig. 3, the quantum calculations of (R)-PVHMA dye and its OPC substrate were achieved from B3LYP/6-31+G(d) method of the Gaussian 09 to investigate the D-A structure on the fluorescence property. For the OPC substrate, most of the electrons mainly concentrate on the aromatic rings in HOMO situation, and they would transfer to ―CHO group in LUMO situation, subsequently, these electrons continually transferred to ―CN group in LUMO situation of (R)-PVHMA dye. As compared with OPC, the more dispersed electrons in LUMO situation of (R)-PVHMA would reduce the energy level difference of HOMO and LUMO situations, which was consistent with the fluorescence emission curves as demonstrated in Fig. S3 (in ESI): The emission wavelength of (R)-PVHMA transferred from 530 nm to 600 nm of OPC with stronger intensity, and the emission wavelength red-shift of (R)-PVHMA dye was very profitable for application in bioimaging area. Otherwise, the PEG-PVH copolymers presented also excellent fluorescence emission in other solvents such as ACN, DCM, DMF and DMSO, as shown in Fig. S4 (in ESI).
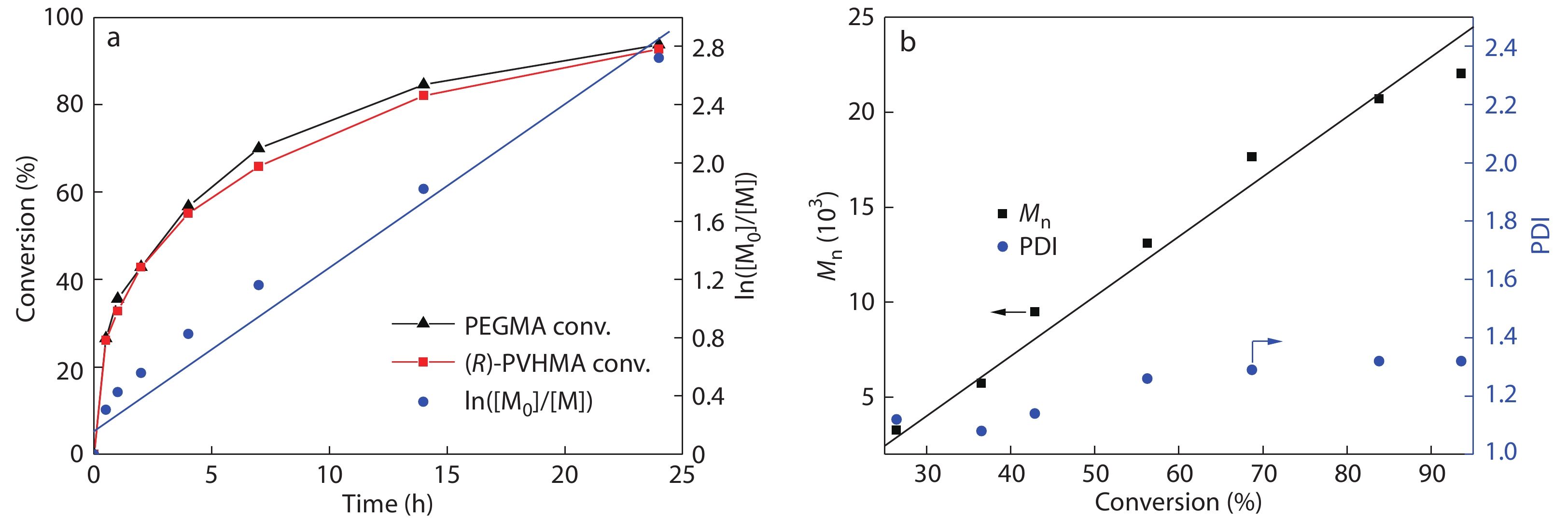
The kinetics analysis of RAFT polymerization was further studied in detail. The 1H-NMR spectra of the samples with various reaction time are exhibited in Fig. S5 (in ESI). The characteristic peaks at 5.61 and 6.17 ppm were attributed to the ―C=CH2 of PEGMA, and the peaks at 5.56 and 6.13 ppm were attributed to the ―C=CH2 of (R)-PVHMA. Otherwise, the typical peak at 6.80 ppm assigned to trimethylbenzene was selected as interior label, and the conversion rate of PEGMA and (R)-PVHMA was calculated by monitoring the intensity change of these peaks mentioned above. From the Fig. S5 (in ESI), it can be observed that the intensity of the above four peaks gradually reduced as the polymerization proceeded, which meant that (R)-PVHMA and PEGMA have been gradually used up in polymerization reactions. For 24 h, their conversion rate was about 90% with a high level. The kinetic study of ln([M0]/[M]) versus time presented a quasilinear first-order procedure as shown in Fig. 4(a). During the polymerization, the conversion rates of (R)-PVHMA and PEGMA remained essentially the same, and the resulted PEG-PVH copolymers should be random copolymers. As shown in Fig. 4(b), during RAFT polymerization, the molecular weights nearly rose linearly with narrow PDI as monomers conversion, indicating a controllable RAFT procedure.
Subsequently, the optical activity of (R)-PVHMA fluorescent monomer and its PEG-PVH2 copolymers was investigated. In the transesterification procedure of HPA and TFEMA, the catalyst Novozym435 played a crucial role. Its high enantioselectivity ultimately led to the preferential consumption of the (R)-HPA enantiomer, which successfully achieved the transformation from racemate (±) HPA to levorotatory (R)-PVHMA. The specific rotation 
Cytotoxicity and Bioimaging Evaluation of PEG-PVH FONs
L929 cells were used as the reference to evaluate the cytotoxicity of the copolymers PEG-PVH. In the CCK-8 assay, L929 cells were subjected to special treatment: incubation with various concentrations of PEG-PVH1 FONs solution (0, 10, 20, 40, 80, 120 μg/mL) for 8 and 24 h.[45−48] As exhibited in Fig. 5(a), the cell viability of each experimental group was above 90%, indicating that the prepared PEG-PVH1 FONs have low cytotoxicity and excellent biocompatibility, conferring the potential biomedical applications of the prepared PEG-PVH1 FONs.
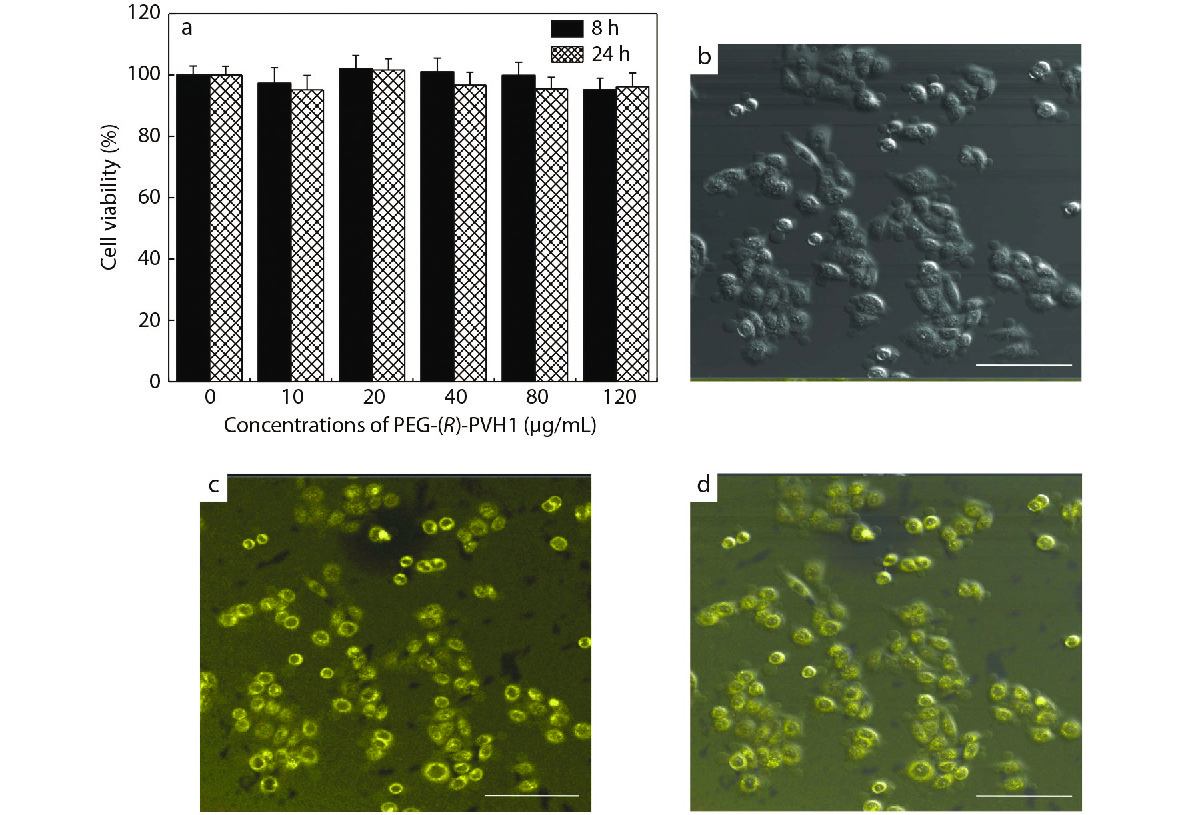
Accordingly, the effect of the prepared PEG-PVH1 FONs on cell imaging was also evaluated with L929 cells as the representative, and they were incubated with PEG-PVH1 FONs solution at a concentration of 20 μg/mL.[49,50] Then, their confocal laser scanning microscope (CLSM) images of experimental cells under 405 nm laser excitation were acquired. As shown in Figs. 5(b)−5(d), the experimental cells showed bright yellow fluorescence under excited light, implying that PEG-PVH1 FONs could readily enter into the cells through endocytosis. On closer inspection, very bright fluorescence could be observed in the periphery of each cell, while the interior was fainter. In the consideration of the sizes of cells, cells cores and PEG-PVH1 FONs, PEG-PVH1 FONs should mainly disperse in the cytoplasmic region after entering the cells. The maximum fluorescence emission wavelength tetraphenylethylene skeleton was generally around 470−510 nm, so the imaging performance of PEG-PVH1 FON was better than them owing to red-shift of the emission wavelength. This was because certain molecules or tissues in the organism would fluoresce on their own, which would interfere with the signal during the imaging process, reduce the sensitivity, and affect the imaging effect. The longer fluorescence wavelength of PEG-PVH1 FONs could reduce the background fluorescence of the organism itself to a certain extent, which also meant that the application prospect of PEG-PVH1 FONs in the field of biomedicine was more prospective.
CONCLUSIONS
Due to the strong electron-donating ability of phenothiazine, it was used as an electron donor to construct fluorescent molecules of red light emission with a D-A structure. Based on phenothiazine, a novel (R)-PVHMA fluorescent monomer with chirality effect was firstly achieved through enzymatic transesterification. The 

Fluorescent light-up probe with aggregation-induced emission characteristics for alkaline phosphatase sensing and activity study
ACS Appl. Mater. Interfaces 2013 5 8784 8789Liang, J.; Kwok, R. T. K.; Shi, H. B.;Tang, B. Z.; Liu, B. Fluorescent light-up probe with aggregation-induced emission characteristics for alkaline phosphatase sensing and activity study. ACS Appl. Mater. Interfaces 2013, 5, 8784−8789.
Full-range intracellular pH sensing by an aggregation-induced emission-active two-channel ratiometricfluorogen
J. Am. Chem. Soc. 2013 135 4926 4929Chen, S. J.; Hong, Y. N.; Liu, Y.; Liu, J. Z.; Leung, C. W. T.; Li M.; Kwok, R. T. K.; Zhao, E. G.; Lam, J. W. Y.; Yu, Y.; Tang, B. Z. Full-range intracellular pH sensing by an aggregation-induced emission-active two-channel ratiometricfluorogen. J. Am. Chem. Soc. 2013, 135, 4926−4929.
Conjugated polyelectrolytes with aggregation-enhanced emission characteristics: synthesis and their biological applications
Chem. Asian J. 2013 8 2436 2445Hu, R. R.; Ye, R. Q.; Lam, J. W. Y.;Li, M.; Leung, C. W. T.; Tang, B. Z. Conjugated polyelectrolytes with aggregation-enhanced emission characteristics: synthesis and their biological applications. Chem. Asian J. 2013, 8, 2436−2445.
NIR emission nanoparticles based on FRET composed of AIE luminogens and NIR dyes for two-photon fluorescence imaging
Chinese J. Polym. Sci. 2019 37 401 408Liu, L. J.; Liu, W.; Ji, G.; Wu Z.Y.; Xu, B.; Qian J.; Tian, W. J. NIR emission nanoparticles based on FRET composed of AIE luminogens and NIR dyes for two-photon fluorescence imaging. Chinese J. Polym. Sci. 2019, 37, 401−408.
The location-influenced fluorescence of AIEgens in the microphase-separatedstructures
Chinese J. Polym. Sci. 2019 37 1060 1064Zhi, Y. F.; Li, C.; Song, Z. H.; Yang, Z. J.; Ma, H. W.; Gao, L. C. The location-influenced fluorescence of AIEgens in the microphase-separatedstructures. Chinese J. Polym. Sci. 2019, 37, 1060−1064.
Bright and photostable organic fluorescent dots with aggregation-induced emission characteristics for noninvasive long-term cell imaging
Adv. Funct. Mater. 2014 24 635 643Qin, W.; Li, K.; Feng, G. X.; Li, M.; Yang, Z. Y.;Liu, B.; Tang, B. Z. Bright and photostable organic fluorescent dots with aggregation-induced emission characteristics for noninvasive long-term cell imaging. Adv. Funct. Mater. 2014, 24, 635−643.
Specific detection of integrin αvβ3 by light-up bioprobe with aggregation-induced emission characteristics
J. Am. Chem. Soc. 2012 134 9569 9572Shi, H. B.; Liu, J. Z.; Geng, J. L.; Tang, B. Z.; Liu, B. Specific detection of integrin αvβ3 by light-up bioprobe with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 9569−9572.
Single-wavelength excited ratiometric fluorescence pH probe to image intracellular trafficking of tobacco mosaic virus
Chinese J. Polym. Sci, 2020 38 587 592Gao, S. J.; Li, Z.; Sun Z. C.; Wen, J. Y.; Li, F. R.; Du, X. Y.; Liu, Y.; Tian, Y.; Niu, Z. W. Single-wavelength excited ratiometric fluorescence pH probe to image intracellular trafficking of tobacco mosaic virus. Chinese J. Polym. Sci, 2020, 38, 587−592.
Mechanofluorochromism of difluoroboron β-ketoiminate boron complexes functionalized with benzoxazole and benzothiazole
Dyes. Pigments 2018 149 276 283Zhao, J. Y.; Peng, J.; Chen, P.; Wang, H. R.; Xue, P. C.; Lu, R. Mechanofluorochromism of difluoroboron β-ketoiminate boron complexes functionalized with benzoxazole and benzothiazole. Dyes. Pigments 2018, 149, 276−283.
Aggregation-induced emission and mechanofluorochromism of tetraphenylbutadiene modified β-ketoiminate boron complexes
Dyes. Pigments 2018 150 165 173Gao, H. Z.; Xu, D. F.; Wang, Y. H.; Zhang, C.; Yang, Y.; Liu, X. L.; Han, A. X.; Wang, Y. Aggregation-induced emission and mechanofluorochromism of tetraphenylbutadiene modified β-ketoiminate boron complexes. Dyes. Pigments 2018, 150, 165−173.
Phenothiazine-based benzoxazole derivates exhibiting mechanochromic luminescence: the effect of a bromine atom
J. Mater. Chem. C 2014 2 3942 3950Xue, P. C.; Yao, B. D.; Sun, J. B.; Xu, Q. X.; Chen, P.; Zhang, Z. Q.; Lu, R. Phenothiazine-based benzoxazole derivates exhibiting mechanochromic luminescence: the effect of a bromine atom. J. Mater. Chem. C 2014, 2, 3942−3950.
Multi-stimuli responsive fluorescent behaviors of a donor-π-acceptor phenothiazine modified benzothiazole derivative
RSC Adv. 2016 6 92144 92151Zhan, Y.; Zhao, J. Y.; Peng, Y.; Ye, W. J. Multi-stimuli responsive fluorescent behaviors of a donor-π-acceptor phenothiazine modified benzothiazole derivative. RSC Adv. 2016, 6, 92144−92151.
Mechanical force-induced luminescence enhancement and chromism of a nonplanar D-A phenothiazine derivative
J. Mater. Chem. C 2016 4 5275 5280Xue, P. C.; Ding, J. P.; Chen, P.; Wang, P. P.; Yao, B. Q.;Sun, J. B.;Lu, R. Mechanical force-induced luminescence enhancement and chromism of a nonplanar D-A phenothiazine derivative. J. Mater. Chem. C 2016, 4, 5275−5280.
Deep-red to near-infrared thermally activated delayed fluorescence in organic solid films and electroluminescent devices
Angew. Chem. Int. Ed. 2017 56 11525 11529Li, C. L.; Duan, R. H.; Liang, B. Y.; Han, G. C.; Wang, S. P.; Ye, K. Q.; Liu, Y.; Yi, Y. P.; Wang, Y. Deep-red to near-infrared thermally activated delayed fluorescence in organic solid films and electroluminescent devices. Angew. Chem. Int. Ed. 2017, 56, 11525−11529.
Naphthothiadiazole-based near-infrared emitter with a photoluminescence quantum yield of 60% in neat film and external quantum efficiencies of up to 3.9% in nondoped OLEDs
Adv. Funct. Mater. 2017 27 1606384Liu, T. X.; Zhu, L. P.; Zhong, C.; Xie, G. H.; Gong, S. L.; Fang, J. F.; Ma, D. G.; Yang, C. L. Naphthothiadiazole-based near-infrared emitter with a photoluminescence quantum yield of 60% in neat film and external quantum efficiencies of up to 3.9% in nondoped OLEDs. Adv. Funct. Mater. 2017, 27, 1606384.
Tuning the AIE activities and emission wavelengths of tetraphenylethene-containing luminogens
Chem. Select. 2016 1 812 818Zhou, J.; He, B. R.; Xiang, J. Y.; Chen, B.; Lin, G. W.; Luo, W. W.; Lou, X. D.; Chen, S. M.; Zhao, Z. J.; Tang, B. Z. Tuning the AIE activities and emission wavelengths of tetraphenylethene-containing luminogens. Chem. Select. 2016, 1, 812−818.
Biocompatible green and red fluorescent organic dots with remarkably large two-photon action cross sections for targeted cellular imaging and real-time intravital blood vascular visualization
ACS Appl. Mater. Interfaces 2015 7 14965 14974Xiang, J. Y.; Cai, X. L.; Lou, X. D.; Feng, G. X.; Min, X. H.; Luo, W. W.; He, B. R.; Goh, C. C.; Ng, L. G.; Zhou, J.; Zhao, Z. J.; Liu, B.; Tang, B. Z. Biocompatible green and red fluorescent organic dots with remarkably large two-photon action cross sections for targeted cellular imaging and real-time intravital blood vascular visualization. ACS Appl. Mater. Interfaces 2015, 7, 14965−14974.
Bright electrochemiluminescence films of efficient aggregation-induced emissionluminogens for sensitive detection of dopamine
Mater. Chem. Front. 2019 3 2051 2057Li, Z. H.; Qin, W.; Wu, J. L.; Yang, Z. Y.; Chi, Z. G.; Liang, G. D. Bright electrochemiluminescence films of efficient aggregation-induced emissionluminogens for sensitive detection of dopamine. Mater. Chem. Front. 2019, 3, 2051−2057.
Imidazole-fused benzothiadiazole-based red-emissive fluorescence probe for lysosomal pH imaging in living cells
Talanta 2020 217 121066Li, L. L.; Li, Y.; Dang, Y. J.; Chen, T. T.; Zhang, A.; Ding, C. Y.; Xu, Z. Imidazole-fused benzothiadiazole-based red-emissive fluorescence probe for lysosomal pH imaging in living cells. Talanta 2020, 217, 121066.
Synthesis of phenanthroimidazole-based four coordinate organoboron compounds
Tetrahedron 2018 74 5819 5825Dhanunjayarao, K.; Sa, S.; Aradhyula, B. P. R.; Venkatasubbajah, K. Synthesis of phenanthroimidazole-based four coordinate organoboron compounds. Tetrahedron 2018, 74, 5819−5825.
Y-shaped dyes based on triphenylamine for efficient dye-sensitized solar cells
Tetrahedron 2012 68 3626 3632Jia, J. H.; Cao, K. Y.; Xue, P. C.; Zhang, Y.; Zhou, H. P.; Lu, R. Y-shaped dyes based on triphenylamine for efficient dye-sensitized solar cells. Tetrahedron 2012, 68, 3626−3632.
Carbazole-based organogel as a scaffold to construct energy transfer arrays with controllable fluorescence emission
Langmuir 2008 24 13730 13735Yang, X. C.; Lu, R.;Xue, P. C.; Li, B.; Xu, D. F.; Xu, T. H.; Zhao, Y. Y. Carbazole-based organogel as a scaffold to construct energy transfer arrays with controllable fluorescence emission. Langmuir 2008, 24, 13730−13735.
Significant improvement of dye-sensitized solar cell performance using simple phenothiazine-based dyes
Chem. Mater. 2013 25 2146 2153Hua, Y.; Chang, S.; Huang, D. D.; Zhou, X.; Zhu, X. J.; Zhao, J. Z.; Chen, T.; Wong, W. Y.; Wong, W. K. Significant improvement of dye-sensitized solar cell performance using simple phenothiazine-based dyes. Chem. Mater. 2013, 25, 2146−2153.
AIEE active novel red-emitting D-π-A phenothiazine chalcones displaying large Stokes shift, solvatochromism and “turn-on” reversible mechanofluorochromism
Dyes. Pigments 2020 181 108539Sachdeva, T.; Milton, M. D. AIEE active novel red-emitting D-π-A phenothiazine chalcones displaying large Stokes shift, solvatochromism and “turn-on” reversible mechanofluorochromism. Dyes. Pigments 2020, 181, 108539.
Water soluble PEGylated phenothiazines as valuable building blocks for bio-materials
Mater. Sci. Eng. C 2020 116 111216Cibotaru, S.; Sandu, A.; Belei, D.; Marin, L. Water soluble PEGylated phenothiazines as valuable building blocks for bio-materials. Mater. Sci. Eng. C 2020, 116, 111216.
From chemistry to nanoscience: not just a matter of size
Angew. Chem. Int. Ed. 2013 52 2678 2683Bai, C. L.; Liu, M. H. From chemistry to nanoscience: not just a matter of size. Angew. Chem. Int. Ed. 2013, 52, 2678−2683.
Emerging chirality in nanoscience
Chem. Soc. Rev. 2013 42 2930 2962Wang, Y.; Xu, J.; Wang, Y. W.; Chen, H. Y. Emerging chirality in nanoscience. Chem. Soc. Rev. 2013, 42, 2930−2962.
Optically active conjugated polymers prepared from achiral monomers by polycondensation in a chiral nematic solvent
Angew. Chem. Int. Ed. 2005 44 4322 4328Goto, H.; Akagi, K. Optically active conjugated polymers prepared from achiral monomers by polycondensation in a chiral nematic solvent. Angew. Chem. Int. Ed. 2005, 44, 4322−4328.
Advances in synthetic optically active condensation polymers-a review
Express. Polym. Lett. 2011 5 142 181Mallakpour, S.; Zadehnazari, A. Advances in synthetic optically active condensation polymers-a review. Express. Polym. Lett. 2011, 5, 142−181.
Sonochemical-assisted fabrication of biologically active chiral poly(ester-imide)/TiO2 bionanocomposites derived from L-methionine and L-tyrosine amino acids
Express Polym. Lett. 2011 5 825 837Mallakpour, S.; Zeraatpisheh, F.; Sabzalian, M. R. Sonochemical-assisted fabrication of biologically active chiral poly(ester-imide)/TiO2 bionanocomposites derived from L-methionine and L-tyrosine amino acids. Express Polym. Lett. 2011, 5, 825−837.
Luminescent pyrenyl-GNA nucleosides: synthesis, photophysics and confocal microscopy studies in cancer HeLa cells
Photochem. Photobiol. Sci. 2019 18 2449 2460Skiba, J.; Kowalczyk, A.; Fik, M. A.; Gapinska, M.; Trzybinski, D.; Wozniak, K.; Vrcek, V.; Czerwieniec, R.; Kowalski, K. Luminescent pyrenyl-GNA nucleosides: synthesis, photophysics and confocal microscopy studies in cancer HeLa cells. Photochem. Photobiol. Sci. 2019, 18, 2449−2460.
Chiral biointerface materials
Chem. Soc. Rev. 2012 41 1972 1984Zhang, M. X.; Qing, G. Y.; Sun, T. L. Chiral biointerface materials. Chem. Soc. Rev. 2012, 41, 1972−1984.
Preparation of a hydroxypropyl-β-cyclodextrin functionalized monolithic column by one-pot sequential reaction and its applicatior for capillary electrochromatographic enantiomer separation
J. Chromatogr. A 2019 1603 269 277Deng, M. D.; Li, S.; Cai,L. Z.; Gou, X. J. Preparation of a hydroxypropyl-β-cyclodextrin functionalized monolithic column by one-pot sequential reaction and its applicatior for capillary electrochromatographic enantiomer separation. J. Chromatogr. A 2019, 1603, 269−277.
Effects of rigid conjugated groups: toward improving enantioseparation performances of chiral porous organic polymers
ACS Appl. Mater. Interfaces 2019 11 37156 37162Tan, H. L.; Chen, Q. B.; Chen, T. T.; Liu, H. L. Effects of rigid conjugated groups: toward improving enantioseparation performances of chiral porous organic polymers. ACS Appl. Mater. Interfaces 2019, 11, 37156−37162.
Chiral polymer-based biointerface materials
Sci. China Chem. 2014 57 540 551Li, M. M.; Qing, G. Y.; Zhang, M. X.; Sun, T. L. Chiral polymer-based biointerface materials. Sci. China Chem. 2014, 57, 540−551.
Combination of enantioselective preparative chromatography and racemization: experimental demonstration and model-based process optimization
Org. Process. Res. Dev. 2018 22 1761 1771Wrzosek, K.; Harriehausen, I.; Seidel-Morgenstern, A. Combination of enantioselective preparative chromatography and racemization: experimental demonstration and model-based process optimization. Org. Process. Res. Dev. 2018, 22, 1761−1771.
End-group effects of piezofluorochromic aggregation-induced enhanced emission compounds containing distyrylanthracene
J. Mater. Chem. A 2012 22 18505 18513Zhang, X. Q.; Chi, Z. G.; Xu, B. J.; Chen, C. J.; Zhou, X.; Zhang, Y.; Liu, S. W.; Xu, J. R. End-group effects of piezofluorochromic aggregation-induced enhanced emission compounds containing distyrylanthracene. J. Mater. Chem. A 2012, 22, 18505−18513.
Branched polymer-protein conjugates made from mid-chain-functional P(HPMA)
Biomacromolecules 2009 10 2847 2851Tao, L.; Liu, J. Q.; Davis, T. P. Branched polymer-protein conjugates made from mid-chain-functional P(HPMA). Biomacromolecules 2009, 10, 2847−2851.
Amphiphilic AIE-active copolymers with optical activity by chemoenzymatic transesterification and RAFT polymerization: synthesis, self-assembly and biological imaging
Dyes. Pigments 2021 184 108829Huang, Z. F.; Chen, Y. L.; Zhou, C. Y.; Wang, K.; Liu, X. B.; Mao, L. C.; Yuan, J. Y.; Tao, L.; Wei, Y. Amphiphilic AIE-active copolymers with optical activity by chemoenzymatic transesterification and RAFT polymerization: synthesis, self-assembly and biological imaging. Dyes. Pigments 2021, 184, 108829.
Fabrication of aggregation induced emission dye-basedfluorescent organic nanoparticles via emulsionpolymerization and their cell imaging applications
Polym. Chem. 2014 5 399 404Zhang, X. Y.; Zhang, X. Q.;Yang, B.; Liu, M. Y.; Liu, W. Y.;Chen, Y. W.; Wei, Y. Fabrication of aggregation induced emission dye-basedfluorescent organic nanoparticles via emulsionpolymerization and their cell imaging applications. Polym. Chem. 2014, 5, 399−404.
Aggregation-induced emission: the whole is more brilliant than the parts
Adv. Mater. 2014 26 5429 5479Mei, J.; Hong, Y. N.; Lam, J. W. Y.;Qin, A. J.; Tang, Y. H.; Tang, B. Z. Aggregation-induced emission: the whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429−5479.
Study of aggregation induced emission of cyano-substituted oligo (p-phenylenevinylene) by femtosecond time resolved fluorescence
Spectrochim. Acta Part A 2011 78 1640 1645Yan, Z. Q.; Yang, Z. Y.; Wang, H.;Li, A. W.; Wang, L. P.; Yang, H.;Gao, B. R. Study of aggregation induced emission of cyano-substituted oligo (p-phenylenevinylene) by femtosecond time resolved fluorescence. Spectrochim. Acta Part A 2011, 78, 1640−1645.
Biocompatible nanoparticles with aggregation-induced emission characteristics as far-red/near-infrared fluorescent bioprobes for in vitro and in vivo imaging applications
Adv. Funct. Mater. 2012 22 771 779Qin, W.; Ding, D.; Liu, J. Z.; Yuan, W. Z.; Hu, Y.; Liu, B.; Tang, B. Z. Biocompatible nanoparticles with aggregation-induced emission characteristics as far-red/near-infrared fluorescent bioprobes for in vitro and in vivo imaging applications. Adv. Funct. Mater. 2012, 22, 771−779.
Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives
J. Phys. Chem. C 2009 113 15845 15853Hu, R. R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J. Z.; Lam, J. W. Y.; Sung, H. H. Y.; Williams, I. D.; Zhong, Y. C.; Wong, K. S.; Pena-Cabrera, E.; Tang, B. Z. Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives. J. Phys. Chem. C 2009, 113, 15845−15853.
A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond
Toxicol. Res. 2012 1 62 68Zhang, X. Y.; Hu, W. B.; Li, J.; Tao, L.; Wei, Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62−68.
Fluorescent PEGylation agent by a thiolactonebased one-pot reaction: a new strategy for theranostic combinations
Polym. Chem. 2014 5 6656 6661Zhao, Y.; Yang, B.; Zhang, Y. L.; Wang, S. Q.; Fu, C. K.; Wei, Y.; Tao, L. Fluorescent PEGylation agent by a thiolactonebased one-pot reaction: a new strategy for theranostic combinations. Polym. Chem. 2014, 5, 6656−6661.
A polymerizable aggregation-induced emission dye for fluorescent nanoparticles: synthesis, molecular structure and application in cell imaging
Polym. Chem. 2019 10 2162 2169Huang Z. F.; Wang R. Z.; Chen Y. L.; Liu X. B.; Wang K.; Mao L. C.; Wang K.; Yuan J. Y.; Zhang X. Y.; Tao L.; Wei Y. A polymerizable aggregation-induced emission dye for fluorescent nanoparticles: synthesis, molecular structure and application in cell imaging. Polym. Chem. 2019, 10, 2162−2169.
Surfactant-dispersed nanodiamond: biocompatibility evaluation and drug delivery applications
Toxicol. Res. 2013 2 335 342Zhang, X. Y.; Wang, S. Q.; Liu, M. Y.; Hui, J. F.; Yang, B.; Tao L.; Wei, Y. Surfactant-dispersed nanodiamond: biocompatibility evaluation and drug delivery applications. Toxicol. Res. 2013, 2, 335−342.
PolyPEGylated nanodiamond for intracellular delivery of a chemotherapeutic drug
Polym. Chem. 2012 3 2716 2719Zhang, X. Y.; Wang, S. Q.; Fu, C. K.; Feng, L.; Ji, Y.; Tao, L.; Li, S. X.; Wei, Y. PolyPEGylated nanodiamond for intracellular delivery of a chemotherapeutic drug. Polym. Chem. 2012, 3, 2716−2719.
Facilely prepared inexpensive and biocompatible self-healing hydrogel: a new injectable cell therapy carrier
Polym. Chem. 2012 3 3235 3238Yang, B.; Zhang, Y. L.; Zhang, X. Y.;Tao, L.; Li, S. X.; Wei, Y. Facilely prepared inexpensive and biocompatible self-healing hydrogel: a new injectable cell therapy carrier. Polym. Chem. 2012, 3, 3235−3238.