INTRODUCTION
In response to the petroleum resource shortage and environmental pollution issues aggravated by the excessive consumption and careless disposal of traditional synthetic plastics, scientists and engineers worldwide have geared continuous efforts to engineer fully biodegradable alternatives derived from 100% biorenewable resources over the past decades.[1-3] Polylactide (PLA) has emerged as an increasingly popular sustainable polyester that can compete with existing commodity plastics in performance (e.g., biocompatibility, mechanical strength, nontoxicity, transparency and processability).[4-6] To data, with the continuous plunge in production cost, PLA has enjoyed great success in some commercial applications, such as biomedicine, packaging and other disposable commodities.[7,8] Unfortunately, the competitive edge of PLA over the common engineering plastics (e.g., poly(butylene terephthalate) (PBT) and nylon) has been considerably hindered by its insufficient heat resistance and durability (associated with the hydrolysis during its service life).[9-12] Owing to the chiral nature of lactic acid used for polymerization, PLA exists as three stereochemical forms including poly(L-lactide) (PLLA), poly(D-lactide) (PDLA), and poly(D,L-lactide) (PDLLA). Fascinatingly, the blending of PLLA and PDLA can form unique stereocomplex crystallites (SCs) by compact side-by-side co-crystallization between the two PLA enantiomers.[13-19] In comparison with enantiopure PLA homocrystals (HCs), the SCs consisting of more compactly packed PLLA/PDLA chains exhibit much higher resistances to heat deformation and hydrolytic degradation.[20-25] Particularly, the melting temperature of SCs (Tm, 220−240 °C) is around 50−60 °C higher than that of HCs, which enables PLA to withstand elevated temperatures of nearly 200 °C.[13] Beyond that, the stereocomplexation is verified to endow the PLLA/PDLA blends with superior mechanical strength and modulus, toughness, and chemical resistance.[26-29] Thus, SC-type PLA (SC-PLA) has attracted growing interests recently due to its tremendous application potential as a next-generation engineering plastic possessing exceptional sustainability and physicochemical properties.
SC-PLA can be easily prepared by either solution or melt mixing of equimolar PLLA and PDLA.[14,15] However, it is extremely challenging to attain useful SC-PLA products through melt-processing of well-stereocomplexed PLLA/PDLA blends possessing high molecular weights (typically Mw≥105 g/mol, which is an essential prerequisite for achieving favorable thermomechanical properties and processability)[30,31] because their SC crystallizability is too weak to stimulate the excusive SC formation during melt-crystallization. In general, large amounts of HCs are preferentially formed in melt-processed products along with the SCs, which undoubtedly gives rise to significantly impaired properties.[31-37] It means that the high performance of SC-PLA materials can hardly be transferred into final products. As such, enhancing the SC crystallizability becomes a vital issue to fully exploit the potential of SC-PLA.[38-40] The weak SC crystallizability has been believed to arise from the insufficient chain mixing between the PLA enantiomers[14,41-45] and inadequate melt stability (i.e., the PLLA/PDLA chain clusters cannot preserve their steteoselective interactions upon melting and then the PLAs chains separated from the clusters could distribute randomly or even demix into PLLA- and PDLA-rich domains).[34-36,46-49] When compared with the blend melts consisting of phase-separated or randomly-distributed PLLA and PDLA chains, the existence of abundant PLLA/PDLA chain clusters makes it easier for the melt to exclusively crystallize into SCs because of the much shorter chain diffusion pathway and lower kinetic barrier.[35,41-50] In this scenario, many strategies have been proposed to enhance the SC crystallizability of SC-PLA by improving the chain mixing degree and/or melt stability, such as stereoblock copolymerization,[51,52] synthesis of special PLAs with complex molecular architectures (e.g., star-shaped and comblike PLAs),[34,50,53-57] selective cross-linking[35] and use of processing additives (i.e., compatibilizers,[47,58] nucleators,[59-62] and plasticizers).[63,64] Most of these approaches involve complicated chain modification reactions or tedious synthesis procedures. Meanwhile, some toxic reactants (e.g., isocyanate) are frequently used. By contrast, the addition of highly efficient compatibilizers into commercial linear high-Mw PLLA/PDLA blends is a simpler, greener and more cost-effective way toward SC-PLA materials with exceptional SC crystallizability. Unfortunately, despite several compatibilizers have been proved to be effective in enhancing the SC crystallizability, their compatibilizing efficiency is rather low. For instance, Pan et al.[50,58] have successfully used the miscible poly(vinyl phenol) (PVPh) and poly(vinyl acetate) (PVAc) to enhance the SC crystallizability of PLLA and PDLA by constructing plentiful hydrogen bonds in the blends, respectively, but at least 30 wt% PVPh or 75 wt% PVAc is needed for the exclusive SC crystallization. Besides, Samuel et al.[65] found that the sole formation of SCs in PLLA/PDLA blends under non-isothermal crystallization conditions can only be realized when the content of miscible poly(methyl methacrylate) (PMMA) is higher than 30 wt%−40 wt%. More seriously, in the current compatibilization strategy for PLLA/PDLA blends, the precious sustainable attributes of PLA are substantially impaired with the incorporation of these petroleum-derived and non-biodegradable compatibilizers. It is therefore highly desirable to develop bio-derived and biodegradable compatibilizers capable of efficiently enhancing the SC crystallizability so as to meet the growing demand for full sustainable SC-PLA products.
Inspired by the compatibilization of immiscible polymer blends using A-B type block copolymers, Fukushima et al.[47] have attempted to use the stereoblock PLA (sb-PLA) as a compatibilizer to enhance the SC formation in racemic PLLA/PDLA blends. It was found that the sb-PLA can markedly suppress the homocrystallization and simultaneously promote the SC crystallization in a certain extent, especially for those with low molecular weights (e.g., Mw=3.9×104 g/mol), indicating a feasible route to prepare all SC-PLA with essentially enhanced SC crystallizability. However, the compatibilizing efficiency of the sb-PLA is also unsatisfactory (the added amount is as high as 30 wt%) probably because the preferential stereocomplexation between adjacent L- and D-blocks makes it difficult for many enantiomeric blocks to collaborate with PLLA and PDLA chains. Moreover, both the melt-processability and end-use properties of SC-PLA could be remarkably deteriorated with the incorporation of large amounts of low-Mw sb-PLA.
To address these challenges, in this work, PDLLA was selected as a potential compatibilizer in the preparation of all SC-PLA with strikingly enhanced SC crystallizability from linear high-Mw PLLA/PDLA blends. It has been verified that the PDLLA is miscible with PLLA or PDLA over the whole composition range,[66] and thereby the intermolecular interactions between the two enantiomers were excepted to be notably enhanced by the PDLLA. Meanwhile, the PDLLA is completely amorphous due to the random distribution of L- and D-lactic acid units,[67,68] which could interact more efficiently with the enantiomeric PLLA/PDLA chains as compared to the sb-PLA possessing strong crystallizability. Both of them are favorable to the enhancement in the melt stability of SC-PLA. Furthermore, the incorporation of PDLLA could facilitate the homogeneous mixing of high-Mw PLLA and PDLA chains during the melt mixing. The isothermal and non-isothermal crystallization behaviors of PLLA/PDLA blends with different contents of PDLLA were examined, with a special attention on the competitive homocrystallization and SC crystallization. The plausible mechanism for the PDLLA-promoted SC crystallization at molecular level is discussed. Also, the important role of the PDLLA molecular weight in tailoring the SC crystallizability of the racemic blends has been highlighted for the first time.
EXPERIMENTAL
Materials
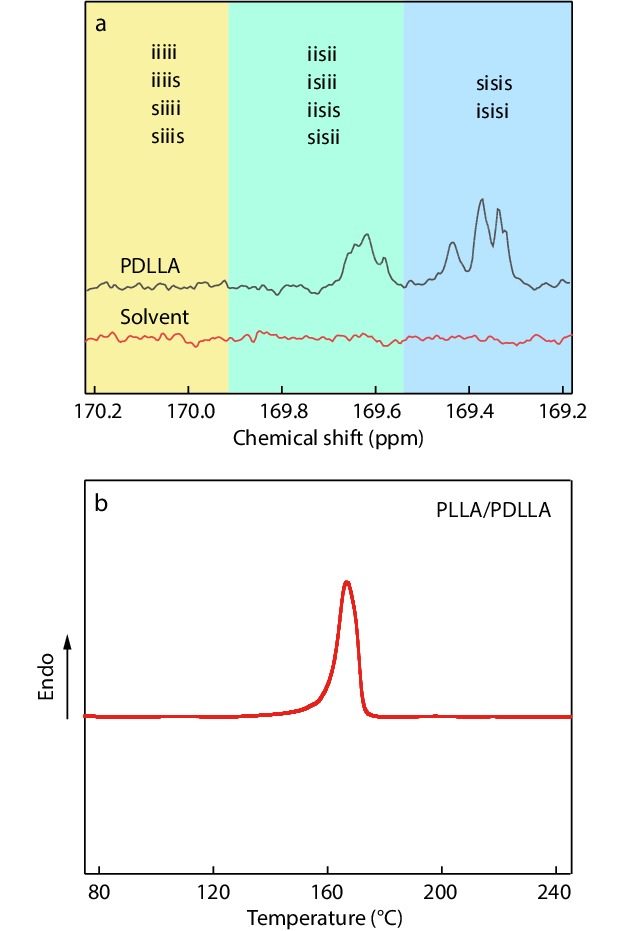
PLLA (Mw=1.7×105 g/mol, PDI=1.7, D-lactic acid content=1.5%) and PDLA (Mw=1.3×105 g/mol, PDI=1.6, L-lactic acid content=0.7%) were purchased from NatureWorks LLC (USA) and Zhejiang Hisun Biomaterial Co., Ltd. (China), respectively. PDLLA with different Mws (between 0.1×105 and 1.5×105 g/mol), synthesized by ring-opening polymerization of D,L-lactide cyclic dimers, was obtained from Jinan Daigang Biomaterial Co., Ltd. (China). Because the sequence distribution of L- and D-lactic acid units is random in the backbones (as confirmed by the exclusive existence of syndiotactic L/D hexads (iisii, isiii, iisis, sisii, sisis, and isisi[36,69,70]) in the 13C-NMR spectra in Fig. 1a), PDLLA does not have the ability to crystallize (Fig. S1 in the electronic supplementary information, ESI). Prior to use, all PLA pellets were dried overnight at 50 °C under vacuum.
Sample Preparation
The PLLA/PDLA (50/50) blends with different amounts of PDLLA (0 wt%−25 wt%) were prepared by solution mixing using dichloromethane (DCM) as a solvent, according to the following protocol. Briefly, the pre-weighted PLLA, PDLA and PDLLA were separately dissolved in DCM (about 20 g/L in concentration) at room temperature and subsequently mixed together under vigorous stirring for at least 1 h. The mixed solution was poured into an excess amount of methanol (used as a precipitation solvent) to give a white flocculent precipitate, followed by the vacuum filtration and vacuum-drying at 50 °C to completely remove the residual solvents. For convenience, the obtained PLLA/PDLA/PDLLA blends were denoted as LD-x, in which x represents the weight fraction of PDLLA. In order to analyze the SC crystallizability between PDLLA and PLLA (or PDLA), PLLA/PDLLA (50/50) binary blends were also prepared using the same mixing procedure.
Characterization
Differential scanning calorimetry (DSC)
DSC analysis was performed on a DSC 8000 calorimeter (PerkinElmer, USA) under a dry nitrogen flow (20 mL/min). The thermal protocols used in different melt-crystallization processes are presented as follows. In the non-isothermal crystallization, the specimens (5−6 mg) were first heated from 30 °C to 250 °C at a scan rate of 10 °C/min and persisted at this temperature for 3 min to erase any thermal history. Then, they were cooled to 30 °C at a rate of 5 °C/min, followed by another heating to 250 °C at 10 °C/min. Specially, this DSC cycle was repeated for three times to examine the melt stability of the blends. In the case of isothermal crystallization, the specimens were quenched (at 150 °C/min) to a pre-determined temperature (100−160 °C) after being melted at 250 °C for 3 min and persisted at this temperature until the crystallization finished. Then, they were reheated to 250 °C at 10 °C/min to analyze the melting behaviors.
The crystallinity values of homocrystallites (




where ΔHm,HC and ΔHm,SC are the measured melting enthalpies of homocrystallites and SCs, respectively; 

The relative fraction of SCs (

Wide angle X-ray diffraction (WAXD)
WAXD measurements were carried out on a X’Pert pro MPD X-ray diffractometer (PANalytical, Holland), which was equipped with a Cu Kα radiation (λ=0.154 nm, 40 kV and 40 mA). The WAXD patterns were recorded in a 2θ range of 5°−40°.
13C nuclear magnetic resonance (13C-NMR)
13C-NMR spectrum (400 MHz) was recorded on a Bruker Avance II-400 MHz NMR spectrometer (Germany) at room temperature, using CDCl3 as solvent.
RESULTS AND DISCUSSION
Miscibility
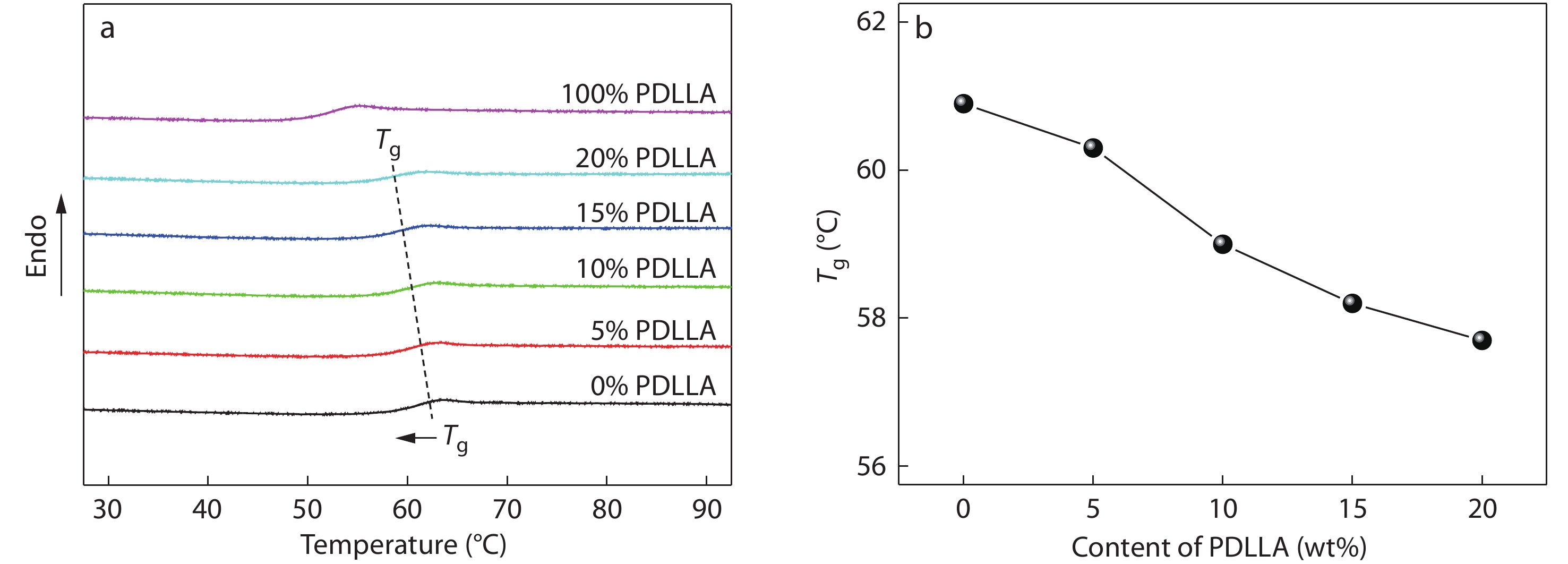
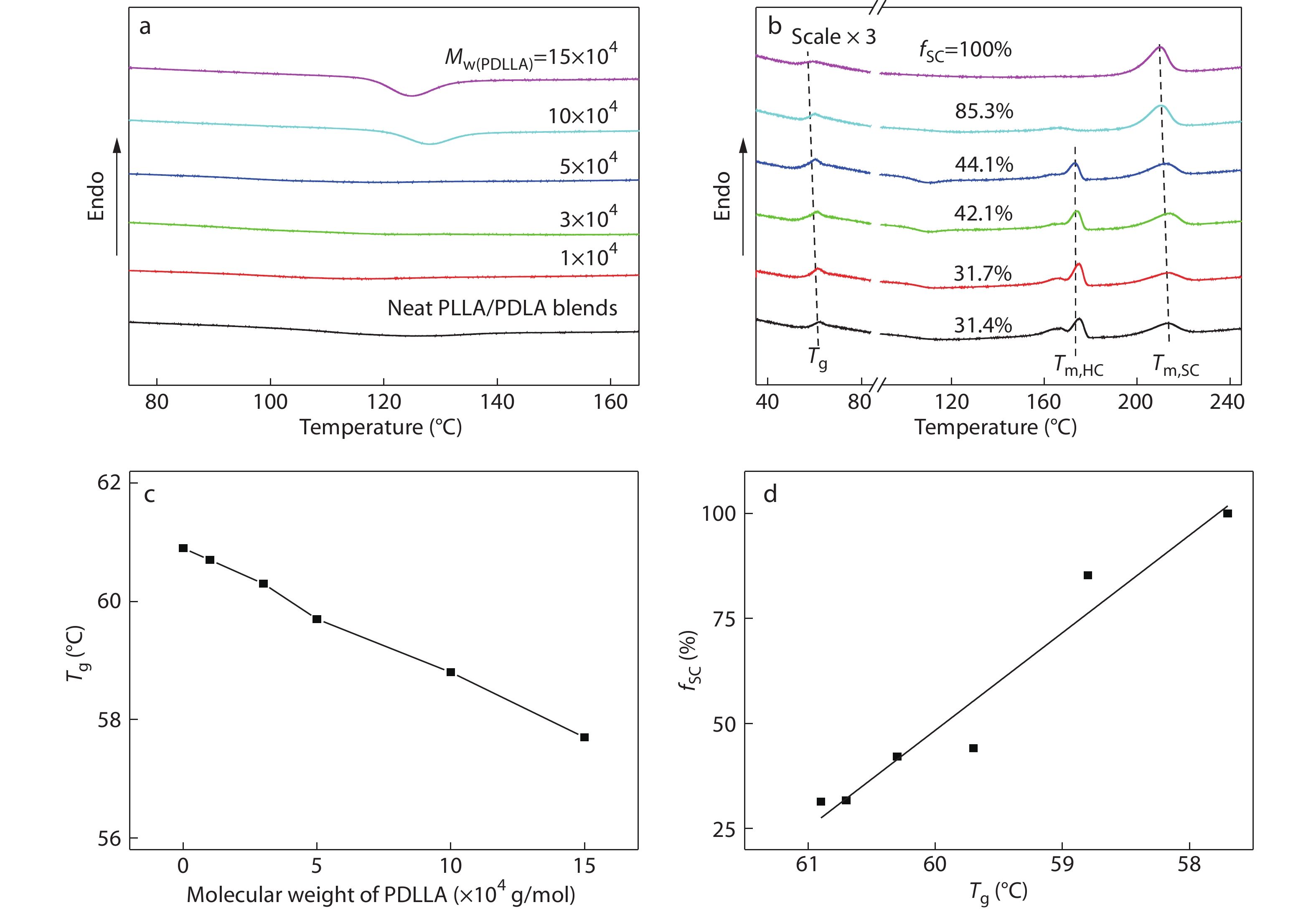
It has been reported that the stereoregularity of PLA has a significant effect on its glass transition temperature (Tg) and the Tg value of PDLLA is much lower than that of PLLA or PDLA,[5,66] so the miscibility between the PDLLA and PLLA/PDLA blend component can be checked by the change of their Tg values upon mixing. Herein, DSC was used as a highly sensitive tool to distinguish the Tg values of the high-Mw blend components. Fig. 2(a) shows the DSC heating curves of amorphous PLLA/PDLA blends with different contents of PDLLA (its Mw is around 1.5×105 g/mol) obtained by complete melting and subsequent ice-water quenching. As expected, all blends exhibit a single and composition-dependent Tg that is located between the Tg values of PDLLA (52.5 °C) and PLLA/PDLA component (60.9 °C), vividly indicating the intermolecular interaction between the PLAs chains is strong and the PLLA/PDLA/PDLLA blends are miscible over the composition range investigated. Meanwhile, the Tg of the blends is found to gradually shift to lower temperature with increasing PDLLA content up to 20 wt% (Fig. 2b). The decreased Tg suggests that the presence of miscible PDLLA could behave as plasticizer to enhance the chain mobility of PLLA and PDLA.
Non-isothermal Crystallization
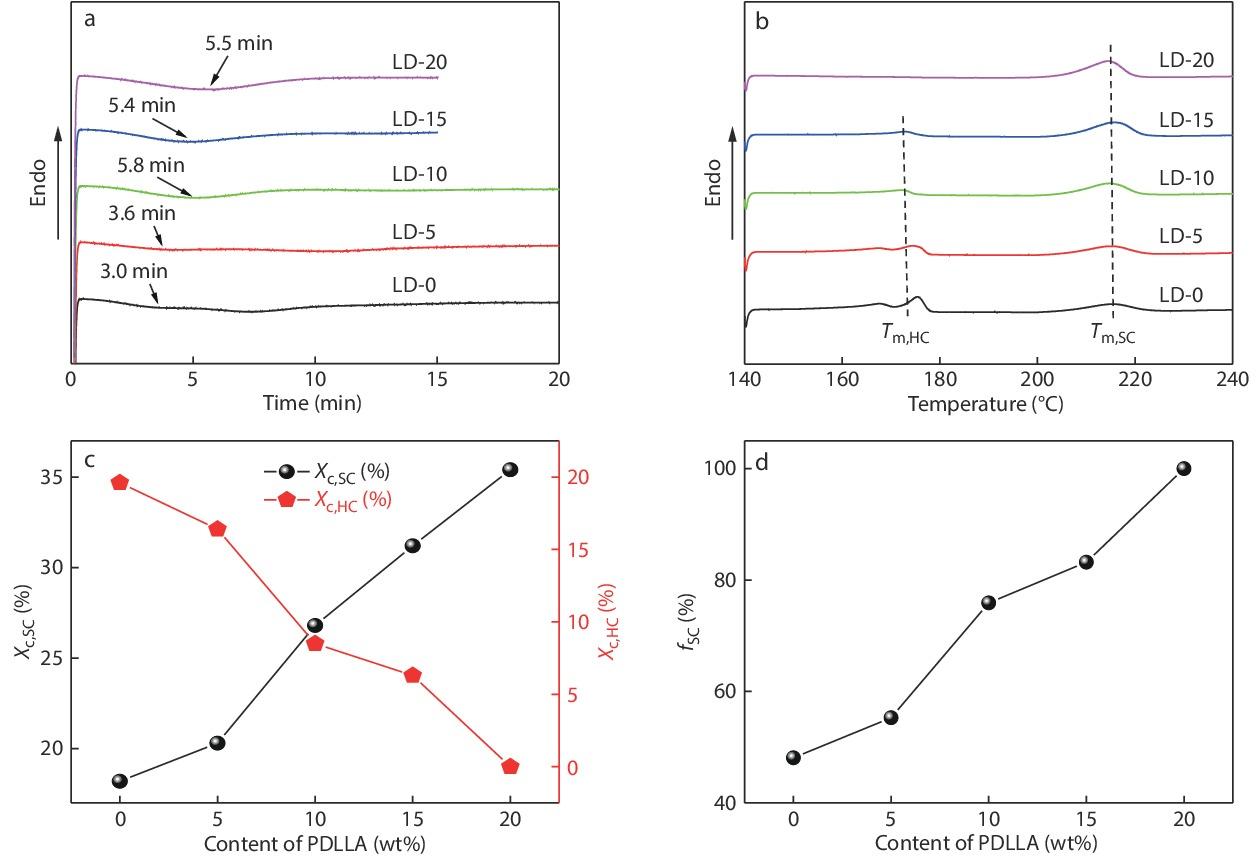
In order to explore the SC crystallizability of high-Mw PLLA/PDLA/PDLLA blends, their melt-crystallization behaviors and kinetics were analyzed under both non-isothermal and isothermal conditions by using DSC and WAXD. Figs. 3(a) and 3(b) present the DSC cooling and subsequent heating curves of PLLA/PDLA blends with different contents of PDLLA, respectively. Based on these DSC results, some important thermal parameters including crystallinity of homocrystallites (












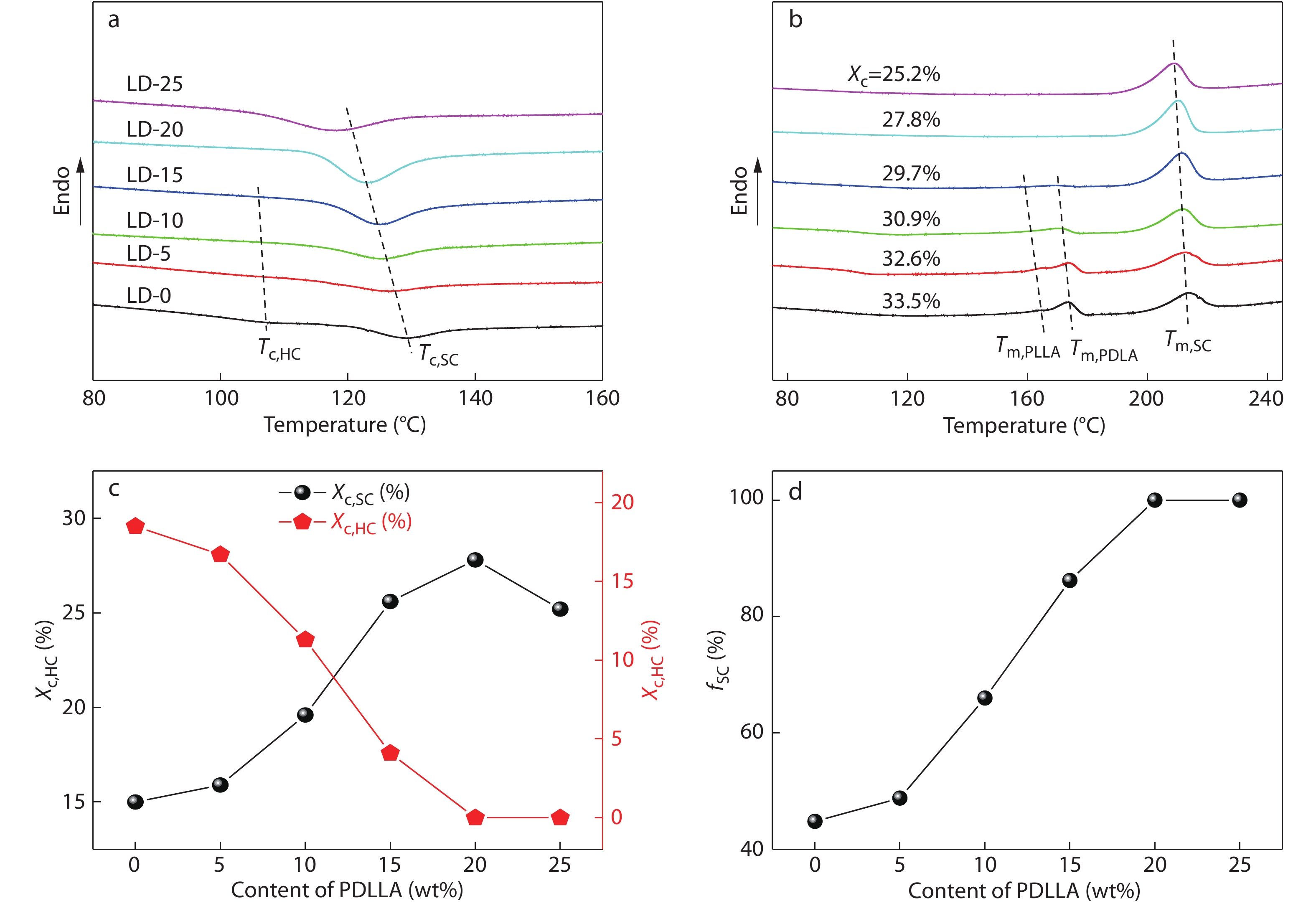
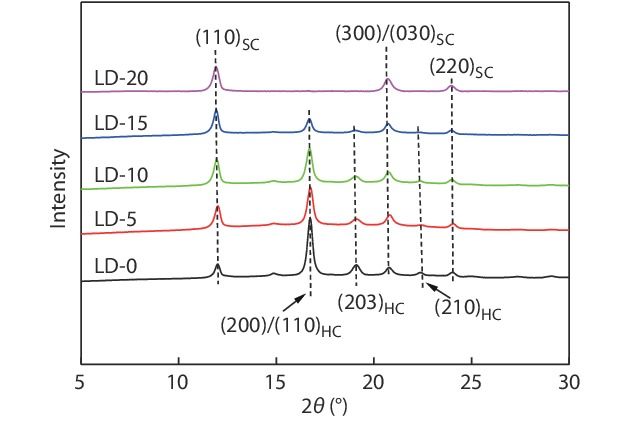
The crystalline composition of PLLA/PDLA/PDLLA blends crystallized under the same non-isothermal condition (i.e., cooling from 250 °C to 50 °C at a rate of 10 °C/min, which was performed by using a Linkam THMS 600 hot stage), was further analyzed by WAXD, and the results are depicted in Fig. 4. In the WAXD patterns, the characteristic diffraction peaks of HCs appear at around 16.9°, 19.0° and 22.3°, while those of SCs locate at around 12.0°, 20.8° and 23.9°.[16] Expectedly, the strong characteristic peaks of HCs as well as the weak peaks of SCs can be observed in the WAXD pattern of the PLLA/PDLA blend without PDLLA, confirming the preferential formation of HCs over SCs. With the increasing PDLA content, the characteristic peak area of HCs decreases sharply and that of SCs increases evidently, which further demonstrates that the HC formation is suppressed and the SC formation is facilitated by the incorporated PDLLA chains at the same time. When the PDLLA content reaches 20 wt%, the characteristic peaks of HCs disappear completely, indicating that the SCs are exclusively formed during the crystallization of the blend. These results are well consistent with the DSC data shown in Fig. 3.
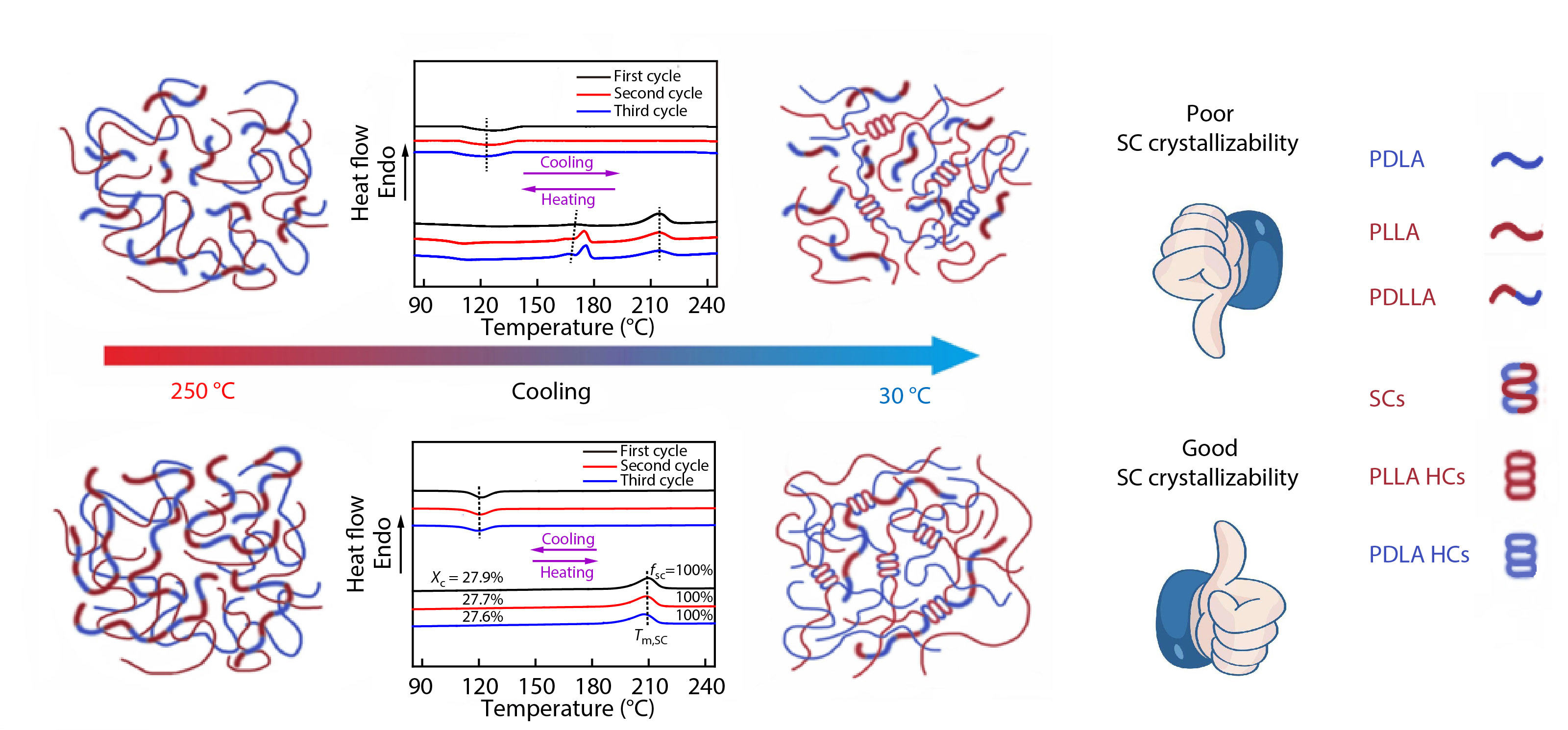
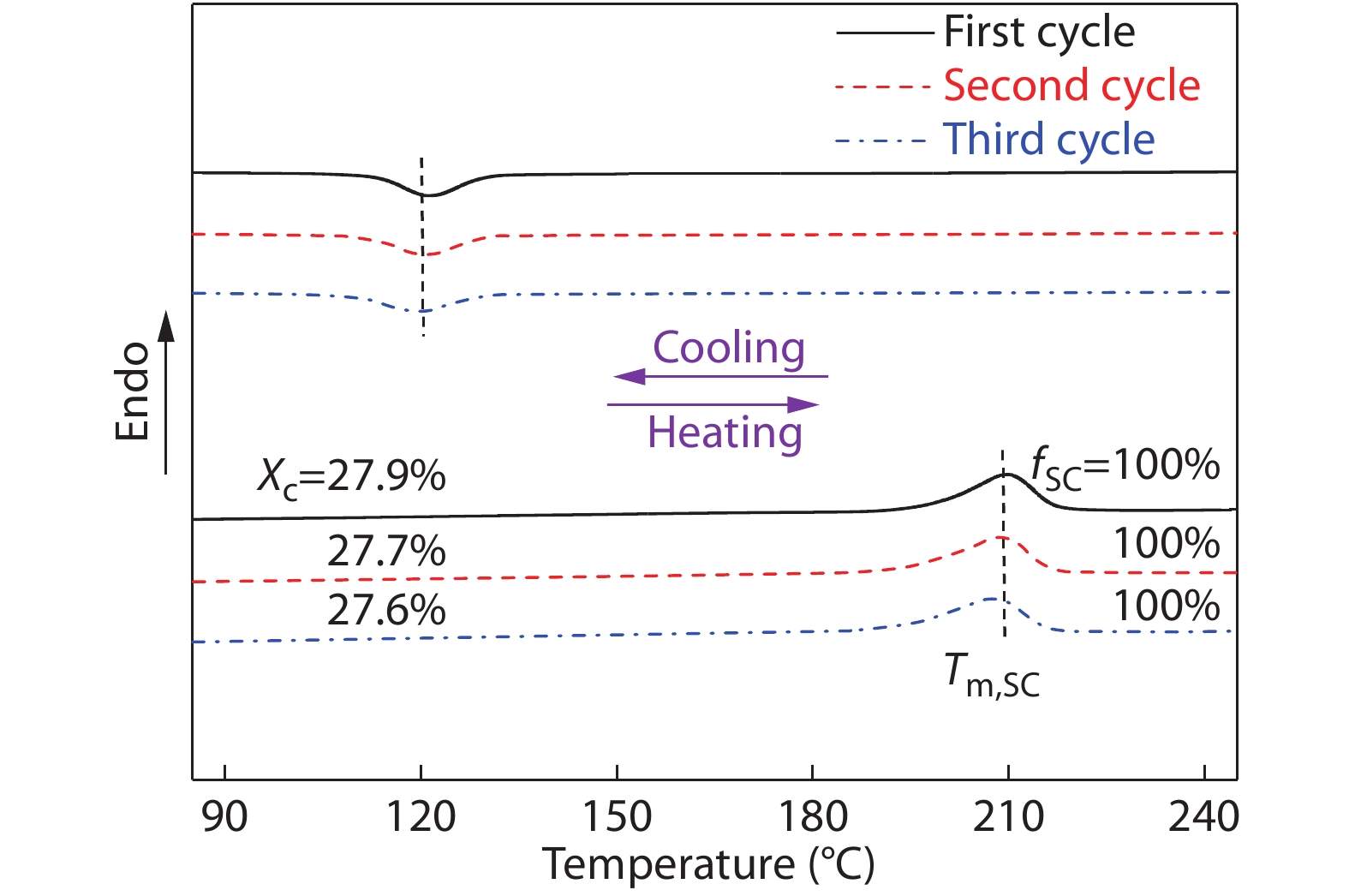
On basis of the above non-isothermal crystallization results, it can be tentatively concluded that incorporating sufficient amounts (e.g., 20 wt%) of high-Mw PDLLA into racemic PLLA/PDLA blends can substantially enhance the SC crystallizability between PLLA and PDLA chains. It also implies that these blends could have good melt stability under non-isotheral crystallization conditions, which can be verified by applying continuous DSC heating/cooling/heating cycles of the PLLA/PDLA blend with 20 wt% PDLLA. As displayed in Fig. 5, the exclusive SC formation is found to be completely reversible during the successive melting and recrystallization processes. More notably, the 


Isothermal Crystallization
Considering that the crystallization temperature (





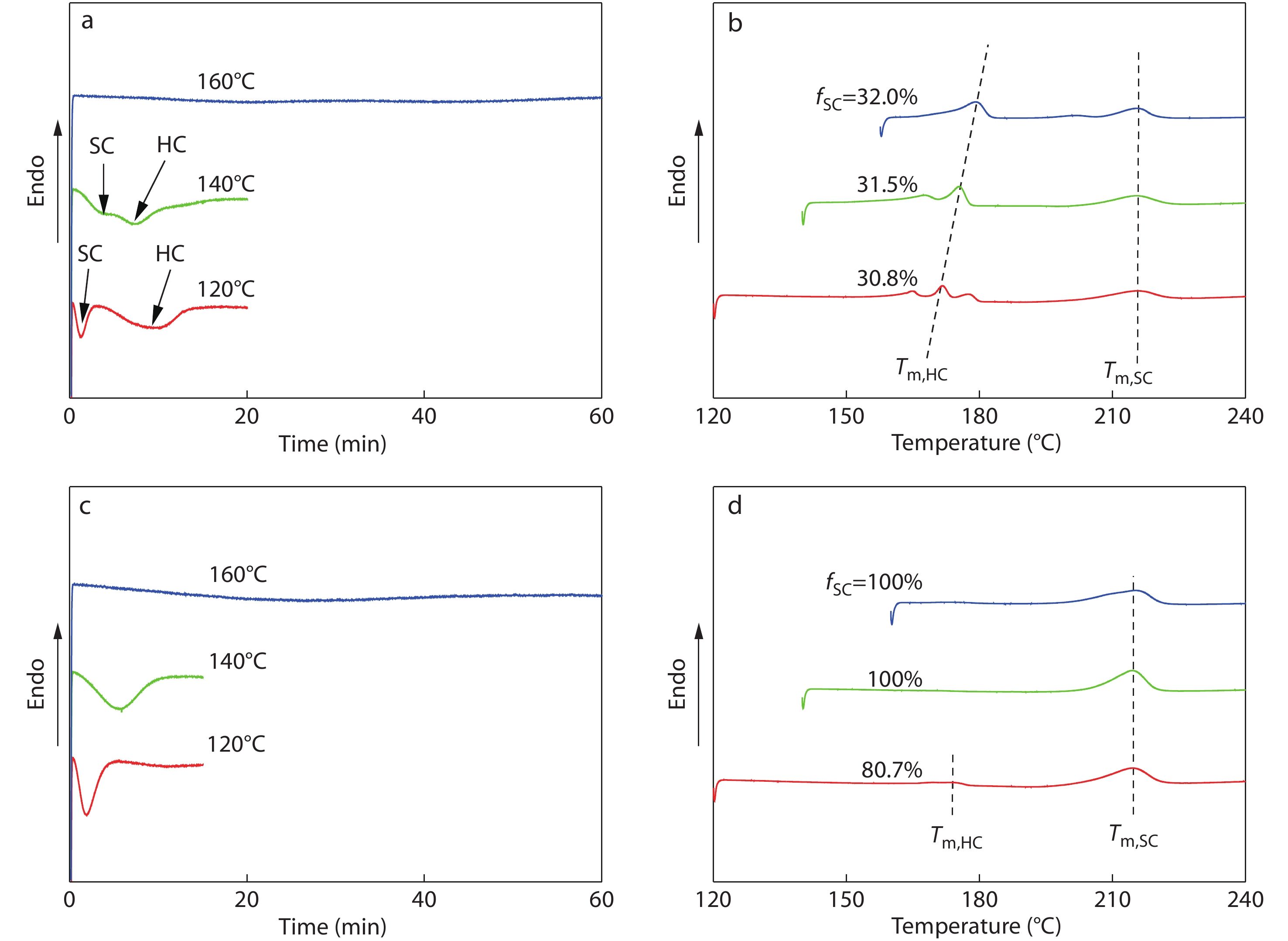
Fig. 7 shows the effect of PDLLA content on the isothermal crystallization behaviors of PLLA/PDLA/PDLLA blends. Obviously, although increasing PDLLA content leads to a slightly decelerated SC crystallization of the blends (the tp is increased from 3.0 min to 5.5 min, Fig. 7a), the 



Strong Dependence of the SC Crystallizability on PDLLA Molecular Weight
Previous studies have proposed that introducing some flexible polymers (e.g., poly(ethylene glycol) (PEG)) can facilitate the SC formation in the melt-crystallization of PLLA/PDLA blends due to the increase in chain mobility.[64] In our blends, the PDLLA induced decrease in the Tg of PLLA/PDLA component (Fig. 2) could be an indicator of the increased chain mobility. In this case, a question arises whether the chain mobility is the main molecular mechanism for the substantially enhanced SC crystallizability and melt stability. Therefore, it is crucial to clarify whether the PDLLA can effectively plasticize the PLLA/PDLA component to reveal the possible mechanisms for the preferred SC crystallization in PLLA/PDLA/PDLLA blends.
It is generally believed that lowering molecular weight of plasticizers is conductive to the plasticizing effect on various polymer matrices.[5] Recently, Yang et al.[64] reported that the enhancement in the SC crystallizability of PLLA/PDLA blends becomes more significant with lowering the Mw of PEG due to the greatly increased segmental mobility of PLAs chains. It is thus natural to think that decreasing PDLLA molecular weight may facilitate the SC crystallization in PLLA/PDLA/PDLLA blends. Fig. 8 presents the strong molecular weight effect on the SC crystallizability during non-isothermal melt-crystallization of the blends containing 20 wt% PDLLA. It is quite unexpected to observe that the SC formation in the melt-crystallization is promoted with increasing Mw of PDLLA and the preferred SC formation can only be achieved when the Mw is higher than 1.0×105 g/mol (Figs. 8a and 8b). More importantly, increasing Mw of PDLLA leads to an evident reduction in the Tg of PLLA/PDLA component (Fig. 8c) and there is a linear relationship between Tg and 

Molecular Mechanism for the PDLLA Induced Substantial Enhancement in SC Crystallizability
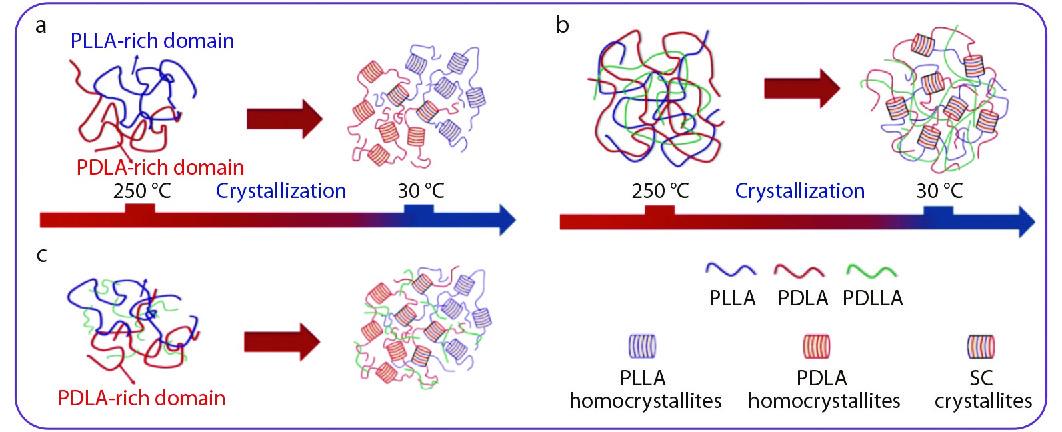
Based on the aforementioned results, it is clear that the incorporation of high-Mw PDLLA can substantially facilitate the SC crystallizability and melt stability of PLLA/PDLA blends due to the increased intermolecular interactions. Fig. 9 illustrates a plausible molecular mechanism for the PDLLA-promoted SC crystallization of high-Mw PLLA/PDLA/PDLLA blends. The intermolecular ordering plays an important role in the crystallization of PLLA/PDLA blends because it can stimulate preferred nucleation and growth of SCs than those of homocrystallites.[71] For the PLLA/PDLA blend without PDLLA, the ordered PLLA/PDLA chain assemblies could be dissociated upon melting, resulting in randomly distributed PLAs chains or even PLLA- and PDLA-rich domains in the blend (Fig. 9a). In this case, the SC crystallization between PLLA and PDLA chains is kinetically limited by the prolonged diffusion pathway from the homogeneous or phase-separated melt to the crystal growth sites upon subsequent crystallization. However, the high-Mw PDLLA can readily entangle with both PLLA and PDLA chains, which is favorable to enhance the intermolecular interactions and improve the stability of the chain assemblies, and thus the heterogeneous blend melts tend to preferentially crystallize into SCs (Fig. 9b). Meanwhile, although the PDLLA does not co-crystallize with PLLA and PDLA, the formation of chain entanglements could also increase the amount of trapped PDLLA chains within amorphous regions of PLLA/PDLA blends and thus facilitate the SC formation in the blends by enhancing chain mobility. In addition, the compatibilizating effect of PDLLA could improve the mixing level between PLLA and PDLA chains, which can shorten the diffusion pathway of the enantiomeric chains in SC formation. With regard to the incorporation of low-Mw PDLLA, the low chain entangleability makes PDLLA difficult to form strong interactions with the enantiomeric PLA chains, which induces a weak promoting effect on SC formation (Fig. 9c).
CONCLUSIONS
In conclusion, a simple-yet-effective strategy has been devised to engineer all SC-PLA with excellent SC crystallizability by incorporating PDLLA into commercial linear high-Mw PLLA/PDLA blends. The single and composition-dependent Tg of the blends suggests that PDLLA has good miscibility with the PLLA/PDLA component. Even though the overall crystallinity is slightly decreased after mixing with PDLLA, the amount of SCs formed in the racemic blends is found to increase remarkably along with the suppression of the homocrystallization under both the non-isothermal and isothermal melt-crystallizations, vividly indicating that the PDLLA can behave as an effective compatibilizer to endow the blends with a much superior SC crystallizability by enhancing the mixing level and intermolecular interactions between PLLA and PDLA chains. More intriguingly, the promoting effect of the PDLLA on SC crystallizability is strongly dependent on its content and molecular weight, and increasing weight fraction and Mw are beneficial to the preferential SC crystallization. In particular, the blends can exclusively crystallize into SCs when the content of high-Mw PDLLA (e.g., Mw=1.5×105) reaches 20 wt%. The higher efficiency of the high-Mw PDLLA in enhancing the SC crystallizability can be reasonably explained by its stronger intermolecular interaction with the PLLA/PDLA chains and the greatly improved chain mixing degree. We believe that these findings could not only provide a valuable way to prepare all SC-PLA engineering plastic suitable for melt processing but also shed light on the fundamental understanding of PDLLA-promoted stereocomplexation between enantiomeric PLAs.
Synthesis of biodegradable polymers from renewable resources
Polym. Chem. 2012 3 836 851Tschan, J. L.; Brulé, E.; Haquette, P.; Thomas, C. M. Synthesis of biodegradable polymers from renewable resources. Polym. Chem. 2012, 3, 836−851.
Biobased plastics and bionanocomposites: current status and future opportunities
Prog. Polym. Sci. 2013 38 1653 1689Reddy, M. M.; Vivekanandhan, S.; Misra, M.; Bhatia, S. K.; Mohanty, A. K. Biobased plastics and bionanocomposites: current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653−1689.
50th Anniversary perspective: there is a great future in sustainable polymers
Macromolecules 2017 50 3733 3750Schneiderman, D. K.; Hillmyer, M. A. 50th Anniversary perspective: there is a great future in sustainable polymers. Macromolecules 2017, 50, 3733−3750.
Processing technologies for poly(lactic acid)
Prog. Polym. Sci. 2008 33 820 852Lim, L. T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820−852.
Poly(lactic acid) crystallization
Prog. Polym. Sci. 2012 37 1657 1677Saeidlou, S.; Huneault, M. A.; Li, H. B.; Park, C. B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657−1677.
Perspective on polylactic acid (PLA) based sustainable materials for durable applications: focus on toughness and heat resistance
ACS Sustain. Chem. Eng. 2016 4 2899 2916Nagarajan, V.; Mohanty, A. K.; Misratt, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899−2916.
Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review
Adv. Drug Deliv. Rev. 2016 107 367 392Farah, S.; Anderson, D. G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367−392.
From lactic acid to poly(lactic acid) (PLA): characterization and analysis of PLA and its precursors
Biomacromolecules 2011 12 523 532Inkinen, S.; Hakkarainen, M.; Albertsson, A. C.; Sodergard, A. From lactic acid to poly(lactic acid) (PLA): characterization and analysis of PLA and its precursors. Biomacromolecules 2011, 12, 523−532.
Durability of polylactide-based polymer blends for injection-molded applications
J. Appl. Polym. Sci. 2013 128 2136 2144Harris, A. M.; Lee, E. C. Durability of polylactide-based polymer blends for injection-molded applications. J. Appl. Polym. Sci. 2013, 128, 2136−2144.
Long-term properties and migration of low molecular mass compounds from modified PLLA materials during accelerated ageing
Polym. Degrad. Stabil. 2012 97 914 920Andersson, S. R.; Hakkarainen, M.; Albertsson, A. C. Long-term properties and migration of low molecular mass compounds from modified PLLA materials during accelerated ageing. Polym. Degrad. Stabil. 2012, 97, 914−920.
An overview of polylactides as packaging materials
Macromol. Biosci. 2004 4 835 864Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835−864.
Significantly improving oxygen barrier properties of polylactide via constructing parallel-aligned shish-kebab-like crystals with well-interlocked boundaries
Biomacromolecules 2014 15 1507 1514Bai, H. W.; Huang, C. M.; Xiu, H.; Zhang, Q.; Deng, H.; Wang, K.; Chen, F.; Fu, Q. Significantly improving oxygen barrier properties of polylactide via constructing parallel-aligned shish-kebab-like crystals with well-interlocked boundaries. Biomacromolecules 2014, 15, 1507−1514.
Stereocomplex formation between enantiomeric poly(lactides)
Macromolecules 1987 20 904 906Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S. H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904−906.
Stereocomplex formation between enantiomeric poly(lactic acid)s. 4. Differential scanning calorimetric studies on precipitates from mixed-solutions of poly(D-lactic acid) and poly(L-lactic acid)
Macromolecules 1991 24 5657 5662Tsuji, H.; Hyon, S. H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 4. Differential scanning calorimetric studies on precipitates from mixed-solutions of poly(D-lactic acid) and poly(L-lactic acid). Macromolecules 1991, 24, 5657−5662.
Stereocomplex formation between enantiomeric poly(lactic acid)s. 9. Stereocomplexation from the melt
Macromolecules 1993 26 6918 6926Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. 9. Stereocomplexation from the melt. Macromolecules 1993, 26, 6918−6926.
Poly(lactic acid) stereocomplexes: a decade of progress
Adv. Drug Deliv. Rev. 2016 107 97 135Tsuji, H. Poly(lactic acid) stereocomplexes: a decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97−135.
Confirmation of the X-ray-analyzed heterogeneous distribution of the PDLA and PLLA chain stems in the crystal lattice of poly(lactic acid) stereocomplex on the basis of the vibrational circular dichroism IR spectral measurement
Macromolecules 2017 50 8066 8071Tashiro, K.; Wang, H. F.; Kouno, N.; Koshobu, J.; Watanabe, K. Confirmation of the X-ray-analyzed heterogeneous distribution of the PDLA and PLLA chain stems in the crystal lattice of poly(lactic acid) stereocomplex on the basis of the vibrational circular dichroism IR spectral measurement. Macromolecules 2017, 50, 8066−8071.
Crystal structure of poly(lactic acid) stereocomplex: Random packing model of PDLA and PLLA chains as studied by X-ray diffraction Analysis
Macromolecules 2017 50 8048 8065Tashiro, K.; Kouno, N.; Wang, H.; Tsuji, H. Crystal structure of poly(lactic acid) stereocomplex: Random packing model of PDLA and PLLA chains as studied by X-ray diffraction Analysis. Macromolecules 2017, 50, 8048−8065.
Stereocomplex crystallization in asymmetric diblock copolymers studied by dynamic monte carlo simulations
Chinese J. Polym. Sci. 2021 39 632 639Xu, Y.; Yang, J.; Liu, Z. F.; Zhou, Z. P.; Nie, Y. J. Stereocomplex crystallization in asymmetric diblock copolymers studied by dynamic monte carlo simulations. Chinese J. Polym. Sci. 2021, 39, 632−639.
Synthesis and thermomechanical properties of stereo triblock polylactides with nonequivalent block compositions
Macromol. Chem. Phys. 2012 213 695 704Masutani, K.; Lee, C. W.; Kimura, Y. Synthesis and thermomechanical properties of stereo triblock polylactides with nonequivalent block compositions. Macromol. Chem. Phys. 2012, 213, 695−704.
Stereocomplexed poly(lactide) composites toward engineering plastics with superior toughness, heat resistance and anti-hydrolysis
Chinese J. Polym. Sci. 2020 38 73 82Wu, B. G.; Yang, W. J.; Niu, D. Y.; Dong, W. F.; Chen, M. Q.; Liu, T. X.; Du, M. L.; Ma, P. M. Stereocomplexed poly(lactide) composites toward engineering plastics with superior toughness, heat resistance and anti-hydrolysis. Chinese J. Polym. Sci. 2020, 38, 73−82.
Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending
Polymer 2003 44 2891 2896Tsuji, H.; Fukui, I. Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending. Polymer 2003, 44, 2891−2896.
Click chemistry synthesis, stereocomplex formation, and enhanced thermal properties of well-defined poly(L-lactic acid)-b-poly(D-lactic acid) stereo diblock copolymers
Polym. Chem. 2017 8 1006 1016Han, L. L.; Xie, Q.; Bao, J. N.; Shan, G. R.; Bao, Y. Z.; Pan, P. J. Click chemistry synthesis, stereocomplex formation, and enhanced thermal properties of well-defined poly(L-lactic acid)-b-poly(D-lactic acid) stereo diblock copolymers. Polym. Chem. 2017, 8, 1006−1016.
Polylactide stereocomplexation leads to higher hydrolytic stability but more acidic hydrolysis product pattern
Biomacromolecules 2010 11 1067 1073Andersson, S. R.; Hakkarainen, M.; Inkinen, S.; Sodergard, A.; Albertsson, A. C. Polylactide stereocomplexation leads to higher hydrolytic stability but more acidic hydrolysis product pattern. Biomacromolecules 2010, 11, 1067−1073.
Sustainable blends of poly(propylene carbonate) and stereocomplex polylactide with enhanced rheological properties and heat resistance
Chinese J. Polym. Sci. 2020 38 1267 1275Li, Y.; Yu, Y. C.; Han, C. Y.; Wang, X. H.; Huang, D. X. Sustainable blends of poly(propylene carbonate) and stereocomplex polylactide with enhanced rheological properties and heat resistance. Chinese J. Polym. Sci. 2020, 38, 1267−1275.
Crystal modulus of poly(lactic acid)s, and their stereocomplex
Polymer 2018 138 124 131Lee, S.; Kimoto, M.; Tanaka, M.; Tsuji, H.; Nishino, T. Crystal modulus of poly(lactic acid)s, and their stereocomplex. Polymer 2018, 138, 124−131.
Mechanical property enhancement of polylactide nanofibers through optimization of molecular weight, electrospinning conditions, and stereocomplexation
Macromolecules 2012 45 5494 5500Zhang, X. W.; Nakagawa, R.; Chan, K. H. K.; Kotaki, M. Mechanical property enhancement of polylactide nanofibers through optimization of molecular weight, electrospinning conditions, and stereocomplexation. Macromolecules 2012, 45, 5494−5500.
Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films
Polymer 1999 40 6699 6708Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699−6708.
Superior performance of fully biobased poly(lactide) via stereocomplexation-induced phase separation: structure versus property
ACS Sustainable Chem. Eng. 2015 3 1470 1478Ma, P. M.; Shen, T. F.; Xu, P. W.; Dong, W. F.; Lemstra, P. J.; Chen, M. Q. Superior performance of fully biobased poly(lactide) via stereocomplexation-induced phase separation: structure versus property. ACS Sustainable Chem. Eng. 2015, 3, 1470−1478.
Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications
Macromol. Biosci. 2005 5 569 597Tsuji, H. Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569−597.
Stereocomplex formation in enantiomeric polylactides by melting recrystallization of homocrystals: crystallization kinetics and crystal Morphology
Macromolecules 2014 47 347 352Na, B.; Zhu, J.; Lv, R. H.; Ju, Y. H.; Tian, R. P.; Chen, B. B. Stereocomplex formation in enantiomeric polylactides by melting recrystallization of homocrystals: crystallization kinetics and crystal Morphology. Macromolecules 2014, 47, 347−352.
Stereocomplex formation of high-molecular-weight polylactide: a low temperature approach
Polymer 2012 53 5449 5454Bao, R. Y.; Yang, W.; Jiang, W. R.; Liu, Z. Y.; Xie, B. H.; Yang, M. B.; Fu, Q. Stereocomplex formation of high-molecular-weight polylactide: a low temperature approach. Polymer 2012, 53, 5449−5454.
Melt stereocomplexation from poly(L-lactic acid) and poly(D-lactic acid) with different optical purity
Polym. Degrad. Stabil. 2013 98 844 852Liu, Y. L.; Sun, J. R.; Bian, X. C.; Feng, L. D.; Xiang, S.; Sun, B.; Chen, Z. M.; Li, G.; Chen, X. S. Melt stereocomplexation from poly(L-lactic acid) and poly(D-lactic acid) with different optical purity. Polym. Degrad. Stabil. 2013, 98, 844−852.
Enhanced melt stability of star-shaped stereocomplexes as compared with linear stereocomplexes
Macromolecules 2006 39 3710 3713Biela, T.; Duda, A.; Penczek, S. Enhanced melt stability of star-shaped stereocomplexes as compared with linear stereocomplexes. Macromolecules 2006, 39, 3710−3713.
Enhancing the melt stability of polylactide stereocomplexes using a solid-state cross-linking strategy during a melt-blending process
Polym. Chem. 2014 5 5985 5993Bai, H. W.; Liu, H. L.; Bai, D. Y.; Zhang, Q.; Wang, K.; Deng, H.; Chen, F.; Fu, Q. Enhancing the melt stability of polylactide stereocomplexes using a solid-state cross-linking strategy during a melt-blending process. Polym. Chem. 2014, 5, 5985−5993.
Unique crystallization behavior of poly(L-lactide)/poly(D-lactide) stereocomplex depending on initial melt states
Polymer 2008 49 5670 5675He, Y.; Xu, Y.; Wei, J.; Fan, Z. Y.; Li, S. M. Unique crystallization behavior of poly(L-lactide)/poly(D-lactide) stereocomplex depending on initial melt states. Polymer 2008, 49, 5670−5675.
Polymer processing via learning from nature and metal metallurgy
Acta Polymerica Sinica (in Chinese) 2016 843 849Bai, D. Y.; Wang, K.; Bai, H. W.; Zhang, Q.; Fu, Q. Polymer processing via learning from nature and metal metallurgy. Acta Polymerica Sinica (in Chinese) 2016, 843−849.
Low-temperature sintering of stereocomplex-type polylactide nascent powder: the role of optical purity in directing the chain interdiffusion and cocrystallization across the particle interfaces
Polymer 2018 150 169 176Bai, D. Y.; Diao, X. Y.; Ju, Y. L.; Liu, H. L.; Bai, H. W.; Zhang, Q.; Fu, Q. Low-temperature sintering of stereocomplex-type polylactide nascent powder: the role of optical purity in directing the chain interdiffusion and cocrystallization across the particle interfaces. Polymer 2018, 150, 169−176.
A promising strategy for fabricating high-performance stereocomplex-type polylactide products via carbon nanotubes-assisted low-temperature sintering
Polymer 2019 162 50 57He, S. W.; Bai, H. W.; Bai, D. Y.; Ju, Y. L.; Zhang, Q.; Fu, Q. A promising strategy for fabricating high-performance stereocomplex-type polylactide products via carbon nanotubes-assisted low-temperature sintering. Polymer 2019, 162, 50−57.
Toward all stereocomplex-type polylactide with outstanding melt stability and crystallizability via solid-state transesterification between enantiomeric poly(L-lactide) and poly(D-lactide)
Polymer 2020 205 122850Li, X. L.; Yang, D. S.; Zhao, Y. B.; Diao, X. Y.; Bai, H. W.; Zhang, Q.; Fu, Q. Toward all stereocomplex-type polylactide with outstanding melt stability and crystallizability via solid-state transesterification between enantiomeric poly(L-lactide) and poly(D-lactide). Polymer 2020, 205, 122850.
An efficient, food contact accelerator for stereocomplexation of high-molecular-weight poly(L-lactide)/poly(D-lactide) blend under nonisothermal crystallization
Polymer 2019 170 54 64Chen, Y.; Hua, W. Q.; Zhang, Z. C.; Xu, J. Z.; Bian, F. G.; Zhong, G. J.; Xu, L.; Li, Z. M. An efficient, food contact accelerator for stereocomplexation of high-molecular-weight poly(L-lactide)/poly(D-lactide) blend under nonisothermal crystallization. Polymer 2019, 170, 54−64.
Molecular simulations of microscopic mechanism of the effects of chain length on stereocomplex formation in polymer blends
Comp. Mater. Sci. 2020 172 109297Xu, Y.; Wu, H. T.; Yang, J.; Liu, R. J.; Nie, Y. J. Molecular simulations of microscopic mechanism of the effects of chain length on stereocomplex formation in polymer blends. Comp. Mater. Sci. 2020, 172, 109297.
Enhanced heat deflection resistance via shear flow-induced stereocomplex crystallization of polylactide systems
ACS Sustain. Chem. Eng. 2017 5 1692 1703Zhang, Z. C.; Sang, Z. H.; Huang, Y. F.; Ru, J. F.; Zhong, G. J.; Ji, X.; Wang, R. Y.; Li, Z. M. Enhanced heat deflection resistance via shear flow-induced stereocomplex crystallization of polylactide systems. ACS Sustain. Chem. Eng. 2017, 5, 1692−1703.
Dynamic Monte Carlo simulations of competition in crystallization of mixed polymers grafted on a substrate
J. Polym. Sci., Part B: Polym. Phys. 2019 57 89 97Nie, Y. J.; Liu, Y. D.; Liu, R. J.; Zhou, Z. P.; Hao, T. F. Dynamic Monte Carlo simulations of competition in crystallization of mixed polymers grafted on a substrate. J. Polym. Sci., Part B: Polym. Phys. 2019, 57, 89−97.
Precursors in stereo-complex crystals of poly(L-lactic acid)/poly(D-lactic acid) blends under shear flow
J. Appl. Crystallogr. 2014 47 14 21Hemmi, K.; Matsuba, G.; Tsuji, H.; Kawai, T.; Kanaya, T.; Toyohara, K.; Oda, A.; Endou, K. Precursors in stereo-complex crystals of poly(L-lactic acid)/poly(D-lactic acid) blends under shear flow. J. Appl. Crystallogr. 2014, 47, 14−21.
The stereocomplex formation and phase separation of PLLA/PDLA blends with different optical purities and molecular weights
Chinese J. Polym. Sci. 2015 33 1713 1720Shao, J.; Liu, Y. L.; Xiang, S.; Bian, X. C.; Sun, J. R.; Li, G.; Chen, X. S.; Hou, H. Q. The stereocomplex formation and phase separation of PLLA/PDLA blends with different optical purities and molecular weights. Chinese J. Polym. Sci. 2015, 33, 1713−1720.
Enhanced stereocomplex formation of poly(L-lactic acid) and poly(D-lactic acid) in the presence of stereoblock poly(lactic acid)
Macromol. Biosci. 2007 7 829 835Fukushima, K.; Chang, Y. H.; Kimura, Y. Enhanced stereocomplex formation of poly(L-lactic acid) and poly(D-lactic acid) in the presence of stereoblock poly(lactic acid). Macromol. Biosci. 2007, 7, 829−835.
Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(L-lactic acid)/poly(D-lactic acid) racemic blends: molecular weight effects
J. Phys. Chem. B 2015 119 6462 6470Pan, P. J.; Han, L. L.; Bao, J. N.; Xie, Q.; Shan, G. R.; Bao, Y. Z. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(L-lactic acid)/poly(D-lactic acid) racemic blends: molecular weight effects. J. Phys. Chem. B 2015, 119, 6462−6470.
Suppression of phase separation for exclusive stereocomplex crystallization of a high-molecular-weight racemic poly(L-lactide)/poly(D-lactide) blend from the glassy state
Macromolecules 2020 53 3493 3503Liu, J. Q.; Qi, X. L.; Feng, Q. J.; Lan, Q. F. Suppression of phase separation for exclusive stereocomplex crystallization of a high-molecular-weight racemic poly(L-lactide)/poly(D-lactide) blend from the glassy state. Macromolecules 2020, 53, 3493−3503.
Competing stereocomplexation and homocrystallization of poly(L-lactic acid)/poly(D-lactic acid) racemic mixture: effects of miscible blending with other polymers
J. Phys. Chem. B 2017 121 6934 6943Bao, J. N.; Xue, X. J.; Li, K.; Chang, X. H.; Xie, Q.; Yu, C. T.; Pan, P. J. Competing stereocomplexation and homocrystallization of poly(L-lactic acid)/poly(D-lactic acid) racemic mixture: effects of miscible blending with other polymers. J. Phys. Chem. B 2017, 121, 6934−6943.
Stereoblock polylactides as high-performance bio-based polymers
Polym. Rev. 2009 49 107 140Kakuta, M.; Hirata, M.; Kimura, Y. Stereoblock polylactides as high-performance bio-based polymers. Polym. Rev. 2009, 49, 107−140.
Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application
Polym. Int. 2006 55 626 642Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application. Polym. Int. 2006, 55, 626−642.
The accelerating effect of the star-shaped poly(D-lactide)-block-poly(L-lactide) stereoblock copolymer on PLLA melt crystallization
CrystEngComm 2016 18 1242 1250Li, W.; Chen, X. Y.; Ma, Y.; Fan, Z. Y. The accelerating effect of the star-shaped poly(D-lactide)-block-poly(L-lactide) stereoblock copolymer on PLLA melt crystallization. CrystEngComm 2016, 18, 1242−1250.
Exclusive stereocomplex crystallization of linear and multiarm star-shaped high-molecular-weight stereo diblock poly(lactic acid)s
J. Phys. Chem. B 2015 119 14270 14279Han, L. L.; Shan, G. R.; Bao, Y. Z.; Pan, P. J. Exclusive stereocomplex crystallization of linear and multiarm star-shaped high-molecular-weight stereo diblock poly(lactic acid)s. J. Phys. Chem. B 2015, 119, 14270−14279.
Rapid stereocomplexation between enantiomeric comb-shaped cellulose-g-poly(L-lactide) nanohybrids and poly(D-lactide) from the melt
Biomacromolecules 2015 16 3723 3729Ma, P. M.; Jiang, L.; Xu, P. W.; Dong, W. F.; Chen, M. Q.; Lemstra, P. J. Rapid stereocomplexation between enantiomeric comb-shaped cellulose-g-poly(L-lactide) nanohybrids and poly(D-lactide) from the melt. Biomacromolecules 2015, 16, 3723−3729.
Stereoblock-like brush copolymers consisting of poly(L-lactide) and poly(D-lactide) side chains along poly(norbornene) backbone: synthesis, stereocomplex formation, and structure-property relationship
Macromolecules 2014 47 7118 7128Isono, T.; Kondo, Y.; Ozawa, S.; Chen, Y.; Sakai, R.; Sato, S.; Tajima, K.; Kakuchi, T.; Satoh, T. Stereoblock-like brush copolymers consisting of poly(L-lactide) and poly(D-lactide) side chains along poly(norbornene) backbone: synthesis, stereocomplex formation, and structure-property relationship. Macromolecules 2014, 47, 7118−7128.
Effect of star-shaped chain architectures on the polylactide stereocomplex crystallization behaviors
Chinese J. Polym. Sci. 2017 35 974 991Zhou, K. Y.; Li, J. B.; Wang, H. X.; Ren, J. Effect of star-shaped chain architectures on the polylactide stereocomplex crystallization behaviors. Chinese J. Polym. Sci. 2017, 35, 974−991.
Stereocomplexation of high-molecular-weight enantiomeric poly(lactic acid)s enhanced by miscible polymer blending with hydrogen bond interactions
Polymer 2016 98 80 87Pan, P. J.; Bao, J. N.; Han, L. L.; Xie, Q.; Shan, G. R.; Bao, Y. Z. Stereocomplexation of high-molecular-weight enantiomeric poly(lactic acid)s enhanced by miscible polymer blending with hydrogen bond interactions. Polymer 2016, 98, 80−87.
Polymorphic crystalline structure and crystal morphology of enantiomeric poly(lactic acid) blends tailored by a self-assemblable aryl amide nucleator
ACS Sustain. Chem. Eng. 2016 4 2680 2688Xie, Q.; Han, L. L.; Shan, G. R.; Bao, Y. Z.; Pan, P. J. Polymorphic crystalline structure and crystal morphology of enantiomeric poly(lactic acid) blends tailored by a self-assemblable aryl amide nucleator. ACS Sustain. Chem. Eng. 2016, 4, 2680−2688.
Stereocomplex crystallization of high-molecular-weight poly(L-lactic acid)/poly(D-lactic acid) racemic blends promoted by a selective nucleator
Polymer 2015 63 144 153Han, L. L.; Pan, P. J.; Shan, G. R.; Bao, Y. Z. Stereocomplex crystallization of high-molecular-weight poly(L-lactic acid)/poly(D-lactic acid) racemic blends promoted by a selective nucleator. Polymer 2015, 63, 144−153.
Supercooling-dependent morphology evolution of an organic nucleating agent in poly(L-lactide)/poly(D-lactide) blends
CrystEngComm 2017 19 1648 1657Shen, S. Q.; Bao, R. Y.; Liu, Z. Y.; Yang, W.; Xie, B. H.; Yang, M. B. Supercooling-dependent morphology evolution of an organic nucleating agent in poly(L-lactide)/poly(D-lactide) blends. CrystEngComm 2017, 19, 1648−1657.
Enantiomeric poly(D-lactide) with a higher melting point served as a significant nucleating agent for poly(L-lactide)
CrystEngComm 2015 17 4334 4342Yin, H. Y.; Wei, X. F.; Bao, R. Y.; Dong, Q. X.; Liu, Z. Y.; Yang, W.; Xie, B. H.; Yang, M. B. Enantiomeric poly(D-lactide) with a higher melting point served as a significant nucleating agent for poly(L-lactide). CrystEngComm 2015, 17, 4334−4342.
Enhanced stereocomplex formation of high-molecular-weight polylactides by gelation in an ionic liquid
Polym. Int. 2014 63 1101 1104Zhu, J.; Na, B.; Lv, R. H.; Li, C. Enhanced stereocomplex formation of high-molecular-weight polylactides by gelation in an ionic liquid. Polym. Int. 2014, 63, 1101−1104.
Enhanced formation of stereocomplex crystallites of high molecular weight poly(L-lactide)/poly(D-lactide) blends from melt by using poly(ethylene glycol)
ACS Sustain. Chem. Eng. 2014 2 2301 2309Bao, R. Y.; Yang, W.; Wei, X. F.; Xie, B. H.; Yang, M. B. Enhanced formation of stereocomplex crystallites of high molecular weight poly(L-lactide)/poly(D-lactide) blends from melt by using poly(ethylene glycol). ACS Sustain. Chem. Eng. 2014, 2, 2301−2309.
Stereocomplexation of polylactide enhanced by poly(methyl methacrylate): improved processability and thermomechanical properties of stereocomplexable polylactide-based materials
Appl. Mater. Inter. 2013 5 11797 11807Samuel, C.; Cayuela, J.; Barakat, I.; Müller, A.; Raquez, J. M.; Dubois, P. Stereocomplexation of polylactide enhanced by poly(methyl methacrylate): improved processability and thermomechanical properties of stereocomplexable polylactide-based materials. Appl. Mater. Inter. 2013, 5, 11797−11807.
Blending effects on polymorphic crystallization of poly(L-lactide)
Macromolecules 2009 42 3374 3380Pan, P. J.; Liang, Z. C.; Zhu, B.; Dong, T.; Inoue, Y. Blending effects on polymorphic crystallization of poly(L-lactide). Macromolecules 2009, 42, 3374−3380.
Crystallization behavior of stereo diblock poly(lactide)s with relatively short poly(D-lactide) segment from partially melted state
Macromol. Mater. Eng. 2015 299 1089 1105Tsuji, H.; Tajima, T. Crystallization behavior of stereo diblock poly(lactide)s with relatively short poly(D-lactide) segment from partially melted state. Macromol. Mater. Eng. 2015, 299, 1089−1105.
Stereocomplex crystallization behavior and physical properties of linear 1-arm, 2-arm, and branched 4-arm poly(L-lactide)/poly(D-lactide) blends: effects of chain directional change and branching
Macromol. Chem. Phys. 2013 214 776 786Sakamoto, Y.; Tsuji, H. Stereocomplex crystallization behavior and physical properties of linear 1-arm, 2-arm, and branched 4-arm poly(L-lactide)/poly(D-lactide) blends: effects of chain directional change and branching. Macromol. Chem. Phys. 2013, 214, 776−786.
Synthesis and properties of high-molecular-weight stereo di-block polylactides with nonequivalent D/L ratios
J. Polym. Sci., Part A: Polym. Chem. 2010 48 794 801Hirata, M.; Kobayashi, K.; Kimura, Y. Synthesis and properties of high-molecular-weight stereo di-block polylactides with nonequivalent D/L ratios. J. Polym. Sci., Part A: Polym. Chem. 2010, 48, 794−801.
Stereoblock poly(lactic acid): synthesis via solid-state polycondensation of a stereocomplexed mixture of poly(L-lactic acid) and poly(D-lactic acid)
Macromol. Biosci. 2005 5 21 29Fukushima, K.; Furuhashi, Y.; Sogo, K.; Miura, S.; Kimura, Y. Stereoblock poly(lactic acid): synthesis via solid-state polycondensation of a stereocomplexed mixture of poly(L-lactic acid) and poly(D-lactic acid). Macromol. Biosci. 2005, 5, 21−29.
Intermolecular ordering as the precursor for stereocomplex formation in the electrospun polylactide fibers
Polymer 2015 60 221 227Zhang, P.; Tian, R. P.; Na, B.; Lv, R. H.; Liu, Q. X. Intermolecular ordering as the precursor for stereocomplex formation in the electrospun polylactide fibers. Polymer 2015, 60, 221−227.