INTRODUCTION
Organic photovoltaics (OPVs) have been investigated deeply and attached much attention as the third generation photovoltaic technology due to its large-area solution processability, mechanical flexibility, light-weight and low-cost.[1−4] Till now, power conversion efficiencies (PCE) of the small-area devices have been improved over 18.2%.[5,6] However, large-area, wearable and flexible OPVs technology has not been developed rapidly in recent years.[7−9] The flexibility of OPVs devices has attracted worldwide attention in recent years. Particularly, Individual wearable electronic components must be flexible, easy processing and low-cost.[10,11] Besides those exploring novel flexible transparent conductive electrodes,[9] researchers have noticed the mechanical flexibility of active layers.[12] Actually, up to now, there are only few reports on the flexibility of the electron transport layers (ETLs).[13] In order to realize the fully-flexible OPVs, it is necessary to put forward the mechanical bendability of the interfacial layer.[14] Recently, the stability of phase separation and flexibility of active layers for OPVs devices has been concerned.[12,14,15] However, the interfacial layer in OPV devices, which provides excitons extraction and charge transfer in the interlayer between the active layer and electrodes, is rarely reported.[16] In fact, solution-processability, mechanical bendability and large-area manufacture technique of interlayer should all be taken into account.[17] The selection of interfacial layer material mainly depends on its energy level and charge transfer characteristics. Thus, the design and synthesis of bendable interfacial layer materials for large-scale flexible organic solar cells device are particularly important. Unfortunately, it is difficult for the widely-used cathode interfacial materials to obtain a desired bending-stability property. Alkali metal salts (such as ZnO, LiF, CsF, Cs2CO3, Li2CO3, etc.)[18−20] are usually accompanied by high-temperature processing and high elastic modulus. Therefore, these kinds of materials are not suitable for large-scale and flexible manufacture. Moreover, the low work function (WF) of metal materials (such as Ca and Ba) also applied for electron transport layer.[18] These metal materials are usually instability in air and high energy consumption evaporation process, which limits their application in large-scale and flexible fabrication. Organic electrolyte materials, due to solution-processing, printablity and low elastic modulus, are the most promising interfacial materials for large-area flexible and printable manufacture in the future.[17]
Conjugated polymeric electrolyte (CPE), composed of a polymeric π-conjugated main chain and polar ionic as side group, was regarded as one of the most efficient organic electrolyte materials. As a result, the CPE possesses unique properties, such as good alcohol-solubility, interface dipole and uniform film formation. Therefore, intersecting solvents can be used in the device fabrication.[21] Moreover, there is a dipole effect between the CPE and electrode. The dipole effect can tune the WF of electrode to match the molecular energy level of acceptor,[22−24] so as to down-shift the charge injection barrier and improve the efficiency of charge collection in the corresponding electrodes.[25,26] In addition, the interfacial layer has also an impact on the vertical phase separation of components of donor and acceptor in blend film, which is conductive to charge carriers selective collection.[27] Therefore, interfacial engineering has significantly influenced the device performances.[28−31] As a result, CPE has been successfully applied to OPVs device with state-of-the-art organic solar cells device. Particularly, Cao et al. have reported a series of CPEs (such as PNDIT-F3N and PNDIT-F3N-Br), which are based on naphthalene-diimide (NDI) conjugated main chain unit with polar groups in the side chain (such as amino and ammonium).[32−34] The NDI-based π-conjugated main chains endow the conjugated copolymers with an excellent electron mobility, resulting in broad application to all-polymer solar cells, organic transistors, organic light emitting diode, battery and perovskite solar cell. Its excellence interfacial modification and unique optoelectronic properties, enabled the devices to exhibit promising efficiency.
In this work, we introduce poly(vinylpyrrolidone) (PVP) into CPE to produce bendable electron transport layers for high-performance OPV devices, in account of the commercially available inexpensive PVP, with characteristics of alcohol-solubility, excellent film-forming property and self-assembly.[35] The devices based on PVP-modified CPE exhibited high device performances and excellent mechanical properties of bendability. The fullerene-free OPVs based on PM6:IT-4F with 2% PVP-modified CPE as ETL yield the best power conversion efficiency (PCE) of 13.42%. More interestingly, a high PCE of 12.59% has been obtained for the fully-flexible OPVs based on PM6:IT-4F. Moreover, the flexible OPVs devices can retain over 80% of its initial PCE after 5000 bending cycles and under various curvature radii of the normalized PCE of device. Obviously, the OPV device based on CPE@PVP as ETL is superior to the bare device based on CPE as ETL. In addition, the PVP-modified CPE device showed a good universality for other active layer systems. Consequently, the best PCEs are 9.30% and 15.2% based on PTB7-Th:PC71BM and PM6:Y6 system, respectively.
EXPERIMENTAL
Preparation of Bendable Interfacial Layer Materials
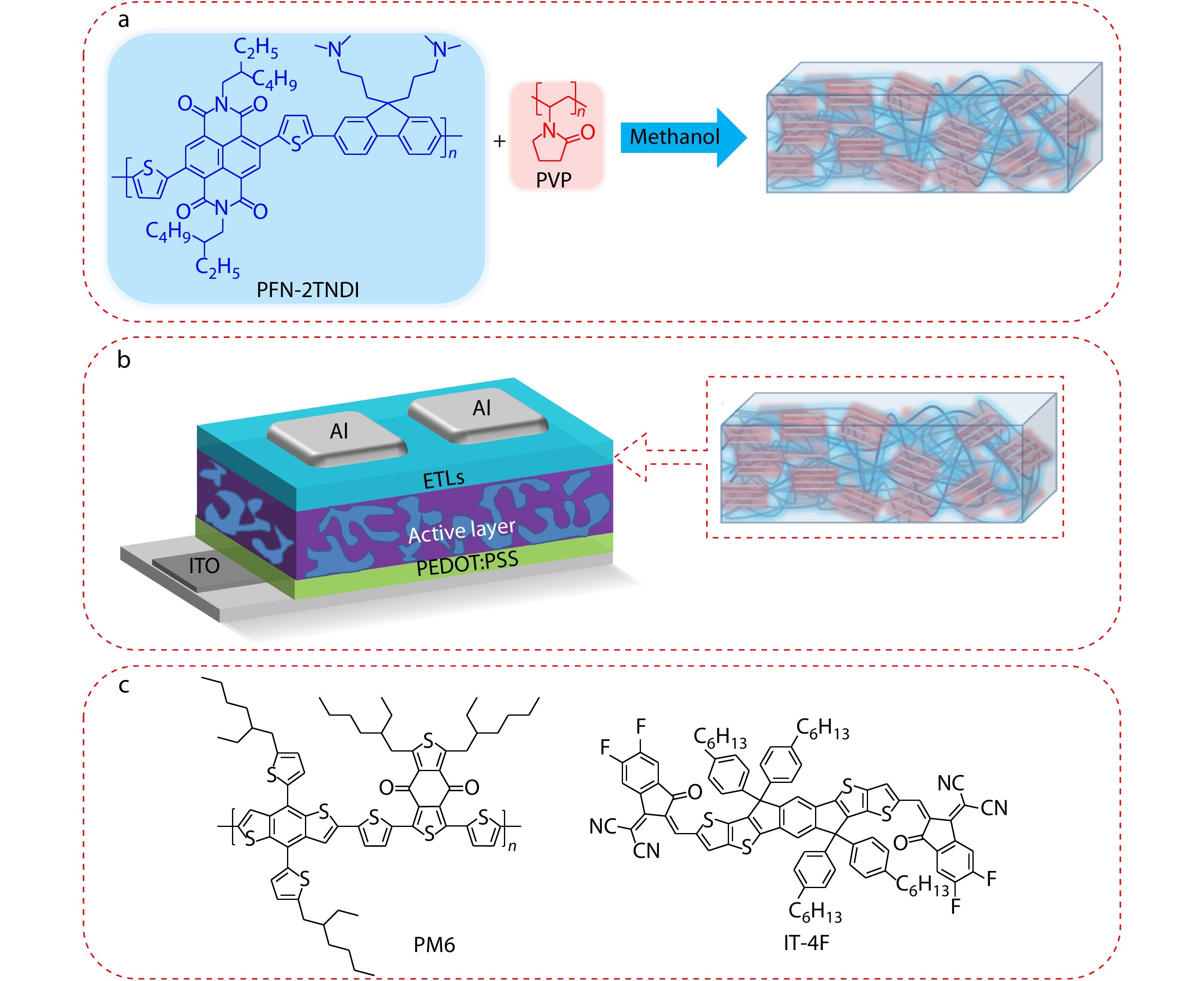
The processing of composite electron transport layer materials composite of interfacial layer materials is shown in Fig. 1(a). The conjugated polyelectrolyte (CPE) is poly[(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-5,5′-bis(2,2′-thiophene)-2,6-naphthalene-1,4,5,8-tetracaboxylic-N,N′-di(2-ethylhexyl)imide] (PNDI-F3N), and both CPE and poly(vinyl pyrrolidone) (PVP) can be easily dissolved in methanol. PNDI-F3N and PVP were mixed and dissolved in methanol in a certain proportion. The molecular weight of the PNDI-F3N, measured by gel permeation chromatography (GPC) using tetrahydrofuran as the eluent, was 13.0 kDa, and PVP with molecular weight of 2000 kDa was purchased from Alfar.
Device Fabrication
Device structure of ITO/PEDOT:PSS/PM6:IT-4F/ETLs/Al
A glass substrate with a pre-patterned ITO (sheet resistance=15 Ω·sq−1) was first ultrasonicated in acetone, detergent, deionized water and isopropanol in turn, and then modified by air plasma treatment for 3 min. After filtration through a 0.45 μm filter, PEDOT:PSS (Bay PVPAI4083, Bayer AG) was spin-coated at 4000 r·min−1 for 45 s to form a thin layer with a thickness of 40 nm on the cleaned ITO substrate, and baked on a hot plate at 140 °C for about 10 min. The PM6:IT-4F (1:1, W/W) was dissolved in chlorobenzene with 0.75 vol% 1,8-diiodoctane (DIO), mixed in solution with total concentration of 20 mg·mL−1, and spin-coated onto the interfacial layer at 2000 r·min−1 for 60 s. The PTB7-Th:PC71BM (1:1.5, W/W) was dissolved in chlorobenzene with 3 vol% 1,8-diiodoctane (DIO), mixed in solution with total concentration of 25 mg·mL−1, and spin-coated onto the interfacial layer at 1000 r·min−1 for 2 min. The PM6:Y6 (1:1.2, W/W) was dissolved in chloroform with 0.5 vol% 1-chloronaphthalene (CN), mixed in solution with total concentration of 17.6 mg·mL−1, and spin-coated onto the interfacial layer at 3000 r·min−1 for 60 s. The cathode materials dissolved in methanol (1 mg·mL−1) was spin-coated on the active layer at 3000 r·min−1 for 60 s. Then the aluminum cathode was thermal evaporation over interface layer under a high vacuum chamber (7×10−4 Pa) to accomplish the device fabrication.
Device structure of PET/ITO/PEDOT:PSSPH1000/ PM6:IT-4F/ ETLs/Al
PET/ITO (sheet resistance=15 Ω·sq−1) was first ultrasonicated in acetone, detergent, deionized water and isopropanol in turn, and then modified by air plasma treatment for 3 min. After filtration through a 0.45 μm filter, PEDOT:PSS (PH1000) was blade-coating to get a thickness of 30 nm thin layer on the cleaned PET/ITO, and baked on a hot plate at 100 °C for about 10 min. The PM6:IT-4F (1:1, W/W) was dissolved in chlorobenzene with 0.75 vol% 1,8-diiodoctane (DIO), mixed in solution with total concentration of 20 mg·mL−1, spin-coated onto the interfacial layer at 2000 r·min−1 for 60 s, and then annealed at 100 °C for 10 min. The cathode materials dissolved in methanol (1 mg·mL−1) was spin-coated on the active layer at 3000 r·min−1 for 60 s. Then the aluminum cathode was thermal evaporation over interface layer under a high vacuum chamber (7×10−4 Pa) to accomplish the device fabrication.
RESULTS AND DISCUSSION
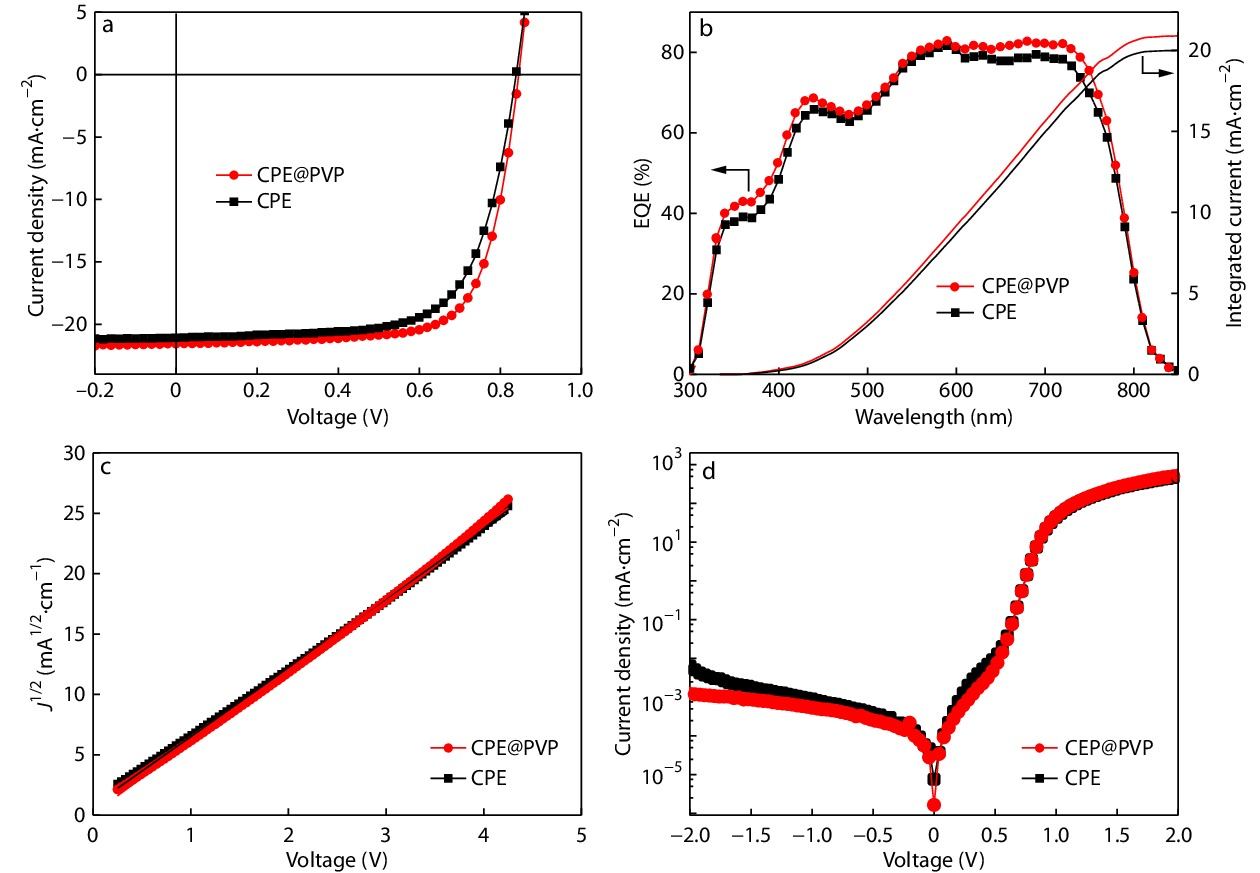
In order to explore the effect of introduction various amounts of PVP into CPEs as ETL on the performance of OPVs devices, the conventional devices structure of ITO/PEDOT:PSS/PM6 (poly[1-(5-(4,8-bis(4-fluoro-5-(2-ethylhexyl) thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophen-2-yl)thiophen-2-yl)-5,7-bis(2-ethylhexyl)-3-(thiophen-2-yl)-4H,8H-benzo[1,2-c:4,5-c']dithiophene-4,8-dione]):IT-4F(3,9-bis(5,6-difluoro-2-methylene-(3-(1,1-dicyanomethylene)-in-danone)-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2',3'-d']-s-indaceno[1,2-b:5,6-b']dithiophene)/ETLs/Ag was fabricated. The detailed information and preparation process of the devices have been provided in the electronic supplementary information (ESI). All ETLs were dissolved in methanol solution and spin-coating on the surface of active layer. The schematic diagram of the device structure is shown in Fig. 1(b). During this process, the bare CPE device was the controlled one. The composite interface layer was incorporating 1%, 2% and 5% PVP into the CPEs, respectively. The optimum J-V characteristic curve is presented in Fig. S1 (in ESI), and the corresponding device parameters are summarized in Table S1 (in ESI). The devices are based on various amounts of PVP incorporating CPE as ETLs, and the active layer is based on PM6:IT-4F to fabricate the devices. All the performances devices with various amounts of PVP modified CPE as ETLs are superior to the controlled device with bare CPE as ETL (PCE of 12.01%, with open-circuit voltage (VOC) of 0.835 V, short-current density (JSC) of 20.94 mA·cm−2, fill factor (FF) of 69.3%). As presented in Fig. 2(a) and Table 1, one of these ETLs, the 2% CPE@PVP shows the best PCE of 13.42%, with VOC of 0.851 V, JSC of 21.57 mA·cm−2 and FF of 73.1%. The corresponding external quantum efficiency (EQE) measurement can confirm the accuracy of JSC. As shown in Fig. 2(b), the integral values calculated by the EQE and JSC based on J-V characteristic curve are kept within the error range of 5%. Furthermore, the electron mobilities are measured by space charge limited current (SCLC) method with the electron-only devices (ITO/ZnO/PM6:IT-4F/ETLs/Al) (Fig. 2c) based on CPE and PVP modified CPE, and the electron mobilities are 1.21×10−3 and 1.33×10−3 cm2·V−1·s−1, respectively. To investigate the difference of device carrier injection and collection based on CPE and CPE@PVP, the J-V characteristic curves of OPVs are measured under dark. As shown in Fig. 2(d), compared to CPE-based OPV, the CPE@PVP-based OPV is obviously suppressed on the side of the reverse current (from −2 V to 0 V). The suppressed dark current indicates that the less charges recombination, consequently contributes to the improvement of JSC and FF.
| Device type | VOC (V) | JSC (mA·cm−2) | FF (%) | PCE (%) |
| a The averages for photovoltaic parameters of each device are given in parentheses with mean variation obtained from 10 devices, and the ± refer to the standard deviation; b Best device PCE; c Values are calculated from EQE. | ||||
| CPE | 0.835±0.012 | 20.98±0.17 (19.85 c) | 69.30±0.23 | 12.01 b (11.8a±0.23) |
| CPE@PVP | 0.851±0.013 | 21.57±0.21 (20.75 c) | 73.11±0.27 | 13.42 b (13.20a±0.22) |
| CPE-flexible OPV | 0.860±0.010 | 16.09±0.19 | 73.84±0.19 | 10.22 b (9.95a±0.24) |
| CPE@PVP-flexible OPV | 0.840±0.011 | 20.61±0.17 | 72.72±0.15 | 12.59 b (12.25a±0.30) |
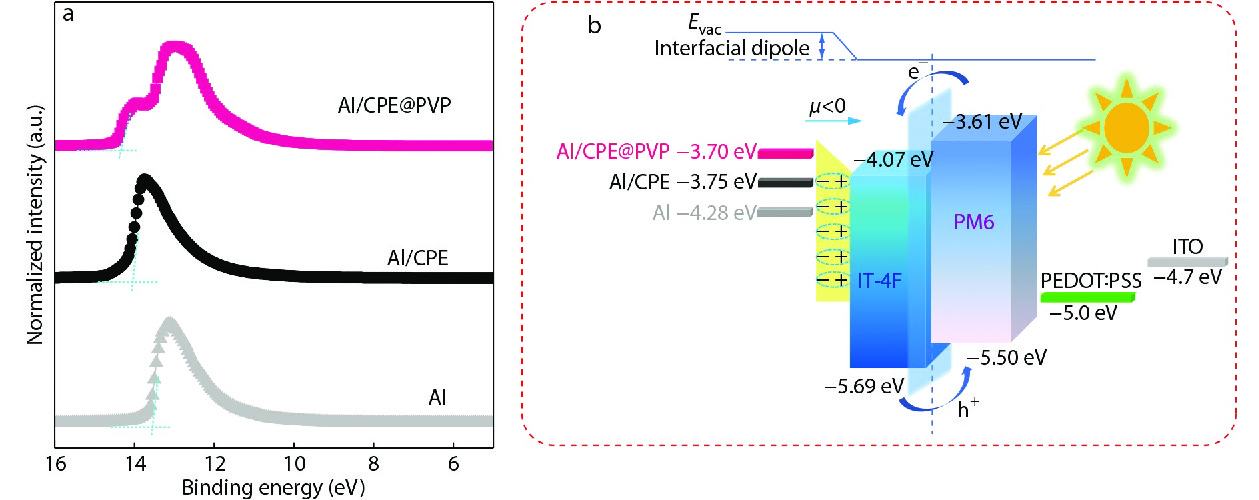
In order to provide a deep insight into the improvement of the device performances, the electrode modified CPE and CPE@PVP are performed to estimate the work function (WF) of Al (cathode) by ultraviolet photoelectron spectroscopy (UPS) test (Figs. 3a and 3b). After deposition of CPE and CPE@PVP on the Al electrode, the WF showed a dramatic decrease. The lower WF can efficiently reduce Schottky barrier and form a suitable energy alignment between the active layer and cathode, consequently improving the electron injection. As for the CPE@PVP modified electrode, the WF showed further decrease. This phenomenon indicated that the amount of PVP incorporating into CPE had an impact on the decrease of its WF. As shown in Fig. 3, compared to the bare CPE/Al (WF=−3.75 eV), the calculated WF of CPE@PVP/Al was reduced to −3.70 eV. The modified Al with lower WF was helpful to alignment energy level between the cathode and acceptor, consequently improving the VOC.
For the conventional device, the upper interfacial layer has little impact on the micromorphology of active layer. However, deposition of ETLs on active layer shows an impact on the contact between cathode and active layer. Fig. S2 and Table S2 (in ESI) show the water contact angle (θ) of interlayer deposited on the PM6:IT-4F blend film, the surface of pristine PM6:IT-4F is hydrophobic. After CPE deposited on the surface of PM6:IT-4F blend film, the surface water contact angle decline to 86.1°, which means that the blend film became more hydrophilic. Replacing the CPE@PVP deposited on the top of PM6:IT-4F blend film, the contact angles decreased to 82.6°. The smaller contact angles mean that more polar groups are accumulated on the top surface of PM6:IT-4F blend film, which would improve the contact with the Al electrode. In addition, the PM6:IT-4F blend film with and without ETLs are measured by atomic force microscopy (AFM) to investigate the morphologies, as shown in Fig. S2 in ESI. Obviously, the pristine PM6:IT-4F blend film showed relatively rough surface with a root mean square (RMS) roughness of 6.08 nm. After deposited CPE and CPE@PVP on the active layer, the RMS decreased to 5.50 and 5.86 nm, respectively. The slightly improved RMS of blend film is conductive to intimate contact between the active layer and Al electrode, in favor of the better device performances.
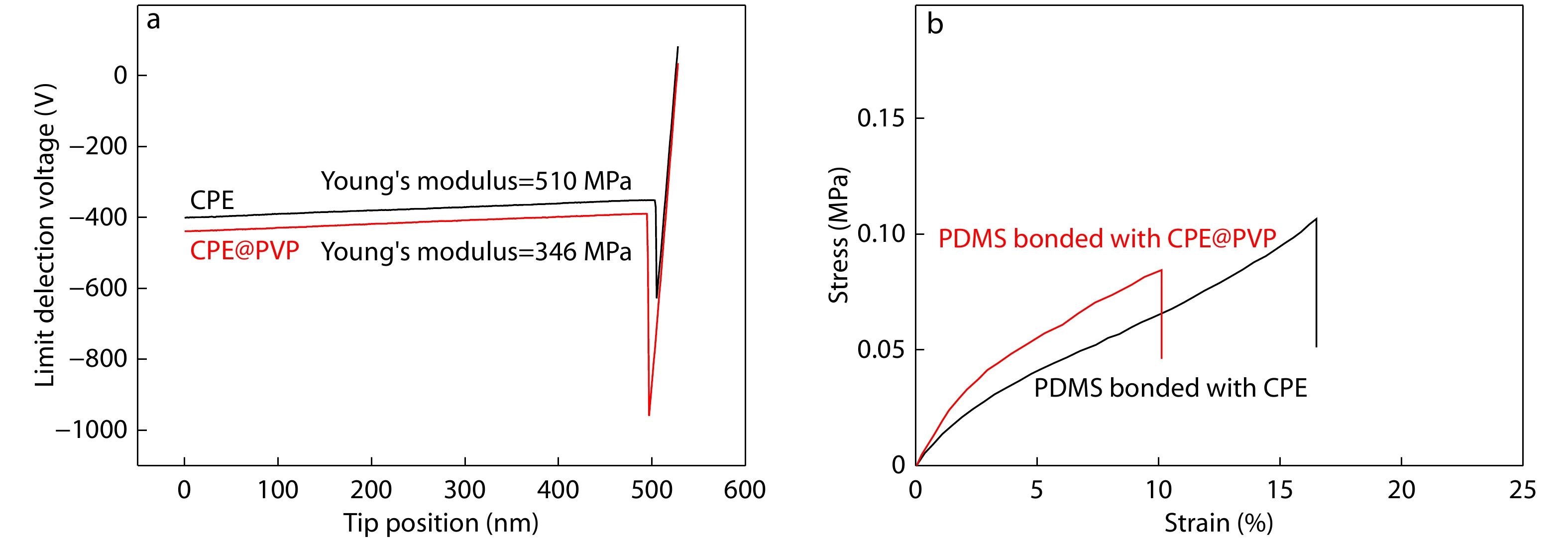
As is well-known, the intrinsic brittleness of interlayer limits its practical application in fully-flexible and wearable OPVs. To verify whether the effect of PVP incorporating into CPE can improve flexural endurance of ETL, the AFM peak-force model was performed to investigate the Young’s modulus. As shown in Fig. 4(a), Young’s modulus value of bare CPE film on glass is 510 MPa, which is higher than that of the CPE@PVP film on glass (346 MPa). This result means that less amount of PVP can significantly improve the mechanical bendability of ETL. After the introduction of PVP, due to its self-assembly and good compatibility with CPE, the aggregation of CPEs can be well dispersed, which is conducive to releasing the stress of the interface layer and obtaining better mechanical bending property. Furthermore, we tested the stress and strain curves bonded on polydimethylsiloxane (PDMS), the CPE@PVP shows more excellent mechanical properties. As shown in Fig. 4(b), the CPE@PVP bonded on PDMS exhibits lower stress under 10% strain curves, which means a better resilience used as the stability of bendability of ETLs for fully-flexible OPV. These results confirm that the incorporation of less PVP additives into CPE could improve mechanical bendability of interface layer.
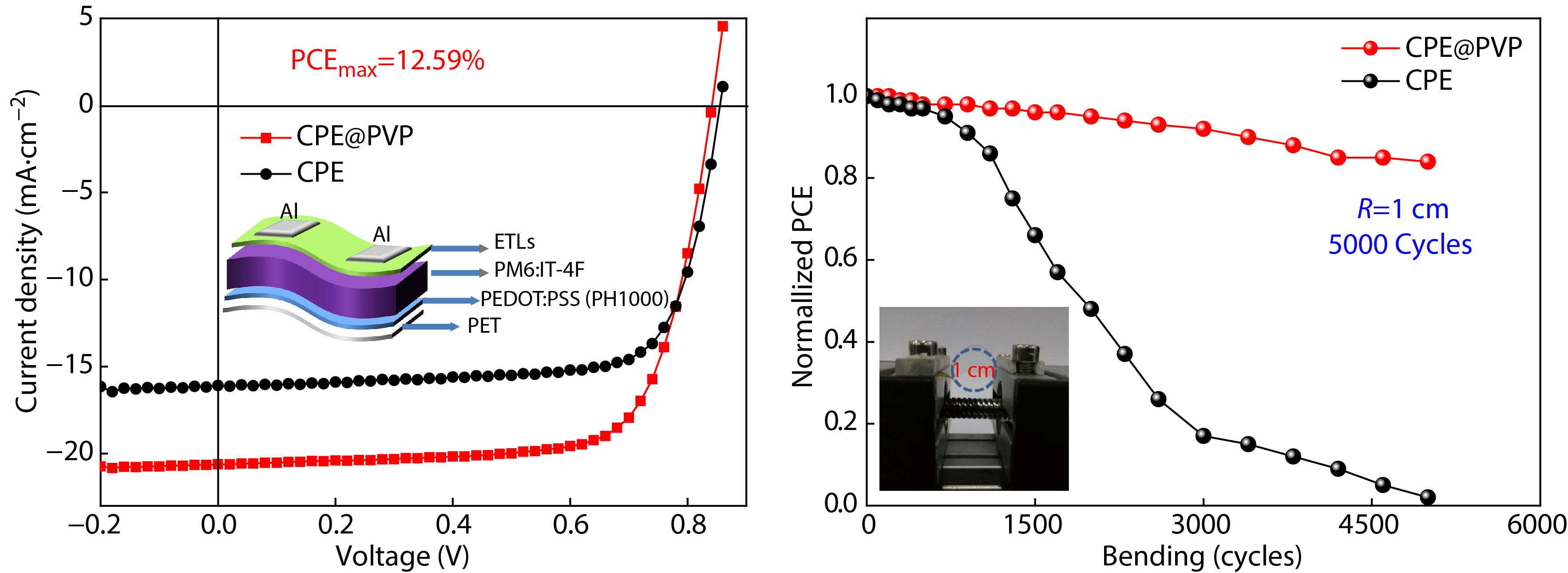
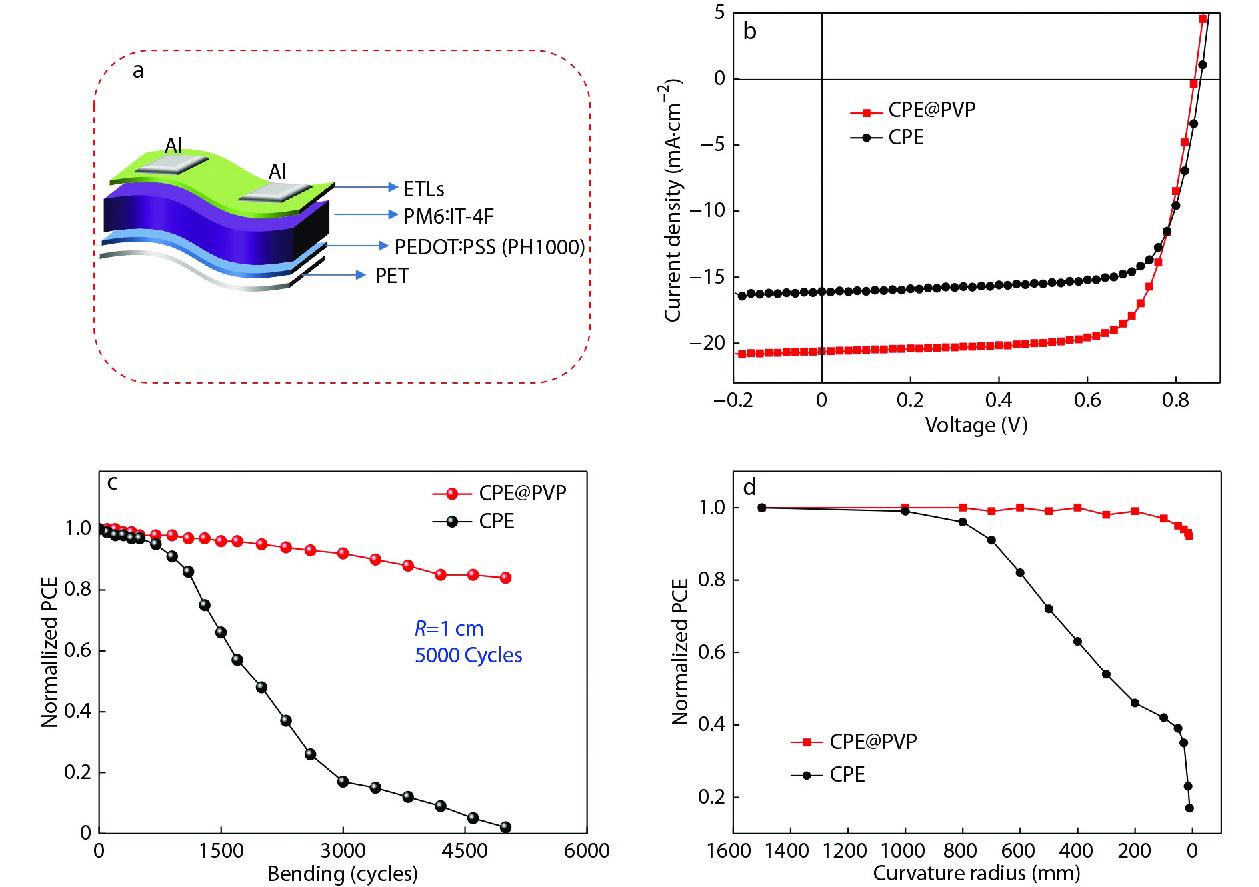
To evaluate the stability of CPE and CPE@PVP based fully-flexible device against external mechanical deformation, we performed the bending cycles test. The fully-flexible OPVs are fabricated with the device structure of PET/PEDOT:PSS(PH1000)/PM6:IT-4F/ETLs/Al, as shown in Fig. 5(a). The PCE based on CPE is 10.22% with a VOC of 0.86 V, a JSC of 16.09 mA·cm−2, an FF of 73.84%, and the efficiency based on CPE@PVP is 12.59%, with a VOC of 0.84 V, a JSC of 20.61 mA·cm−2, an FF of 70.72% (Fig. 5b). The normalized PCEs and under 5000 bending cycles with a radius of 1 cm of the corresponding device as a function are shown in Fig. 5(c). The CPE@PVP based fully-flexible device retains over 80% of its initial PCE. Furthermore, as shown in Fig. 5(d), various bending tests with curvature radius ranging from 0 mm to 1500 mm were carried out, and the CPE@PVP is superior to the bare CPE modified devices.
To investigate the universality of CETLs, the devices based on different active layer systems were exploited. The conventional device based on poly[[4,8-bis(5-(2-ethylhexyl) thiophen-2-yl)-6-methyl]benzo[1,2-b:4,5-b]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl]] (PTB7-Th) as a donor and [6,6]-phenyl-C71-butylic acid methyl ester (PC71BM) as an acceptor were fabricated. The corresponding J-V characteristics and device parameters are summarized in Fig. S3 and Table S2 in ESI. The ETL based on CPE@PVP as cathode interlayer yield an excellent PCE of 9.30%, higher than that of bare CPE (8.38%). And the increased PCEs of the CPE@PVP modified device are mainly related to the VOC, JSC and FF. Besides, we also applied the new composite interface layer in PM6:Y6 ((2,2'-((2Z,2'Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2,"3":4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b]indole-2,10-diyl)bis(me-thanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile)) as active layer system with conventional device. A best PCE of 15.20% with a VOC of 0.832 V, a JSC of 25.10 mA·cm−2, and an FF of 72.08% was achieved for the device based on CPE@PVP. Compared to the control devices, both of the CPE@PVP modified OPVs present enhanced device performances, mainly due to the improvement of VOC, JSC and FF values, which are consistent with the PM6:IT-4F-based device. These results indicated that the cathode interlayer via introduced by PVP into CPE exhibits a good universality for other high-performance active layer systems. Therefore, the CPE@PVP is a promising interfacial material for large-area flexible and printable manufacture in the future.
CONCLUSIONS
In conclusion, we synthesized a bendable ETL by incorporating PVP into CPE and applying it to high-performance OPVs. The devices based on PVP-modified CPE exhibited higher efficiency and more excellent mechanical properties of bendability. As a result, the PVP-modified CPE obtained the best PCE of 13.42%, based on PM6:IT-4F. More importantly, an excellent PCE of 12.59% was obtained for the fully-flexible OPVs. As far as we know, this is one of the highest PCEs for fully-flexible OPV based PM6:IT-4F system. Furthermore, the fully-flexible OPV devices based on CPE@PVP can retain more than 80% of its initial PCE after 5000 bending cycles and the mechanical properties of device are superior to those of the barely CPE as ETLs under various curvature radii. These findings indicate that these composite interfacial materials are promising candidates as electron transport layer for flexible printable organic photovoltaic in the near future.
Polymer power
Nat. Photon. 2009 3 447 449Shrotriya, V. Polymer power. Nat. Photon. 2009, 3, 447−449.
A general approach for lab-to-manufacturing translation on flexible organic solar cells
Adv. Mater. 2019 31 1903649Meng, X. C.; Zhang, L.; Xie, Y. P.; Hu, X. T.; Xing, Z.; Huang, Z. Q.; Liu, C.; Tan, L. C.; Zhou, W. H.; Sun, Y. M.; Ma, W.; Chen, Y. W. A general approach for lab-to-manufacturing translation on flexible organic solar cells. Adv. Mater. 2019, 31, 1903649.
Solution-processed infrared photovoltaic devices with > 10% monochromatic internal quantum efficiency
Appl. Phys. Lett. 2005 87 213112Maria, A.; Cyr, P. W.; Klern, E. J. D.; Levina, L.; Sargent, E. H. Solution-processed infrared photovoltaic devices with > 10% monochromatic internal quantum efficiency. Appl. Phys. Lett. 2005, 87, 213112.
18% Efficiency organic solar cells
Sci. Bull. 2020 65 272 275Liu, Q. S.; Jiang, Y. F.; Jin, K.; Qin, J. Q.; Xu, J. G.; Li, W. T.; Xiong, J.; Liu, J. F.; Xiao, Z.; Sun, K.; Yang, S. F.; Zhang, X. T.; Ding, L. M. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272−275.
Self-assembled monolayer enables hole transport layer-free organic solar cells with 18% efficiency and improved operational stability
ACS Energy Lett. 2020 5 2935 2944Lin, Y. B.; Firdaus, Y.; Isikgor, F. H.; Nugraha, M. I.; Yengel, E.; Harrison, G. T.; Hallani, R.; El-Labban, A.; Faber, H.; Ma, C.; Zheng, X. P.; Subbiah, A.; Howells, C. T.; Bakr, O. M.; McCulloch, I.; de Wolf, S.; Tsetseris, L.; Anthopoulos, T. D. Self-assembled monolayer enables hole transport layer-free organic solar cells with 18% efficiency and improved operational stability. ACS Energy Lett. 2020, 5, 2935−2944.
Large-scale flexible and highly conductive carbon transparent electrodes via roll-to-roll process and its high performance lab-scale indium tin oxide-free polymer solar cells
Chem. Mater. 2014 26 6293 6302Hu, X. T.; Chen, L.; Zhang, Y.; Hu, Q.; Yang, J. L.; Chen, Y. W. Large-scale flexible and highly conductive carbon transparent electrodes via roll-to-roll process and its high performance lab-scale indium tin oxide-free polymer solar cells. Chem. Mater. 2014, 26, 6293−6302.
Large-scale ultra-adhesive and mechanically flexible silver grids transparent electrodes by solution process
Org. Electron. 2018 61 296 303Wang, Q. X.; Hu, X. T.; Yang, X.; Liu, G. L.; Meng, X. C.; Xie, Y. P.; Xiao, Y. B.; Liu, J. L.; Tan, L. C.; Chen, Y. W. Large-scale ultra-adhesive and mechanically flexible silver grids transparent electrodes by solution process. Org. Electron. 2018, 61, 296−303.
Large-scale stretchable semiembedded copper nanowire transparent conductive films by an electrospinning template
ACS Appl. Mater. Interfaces 2017 9 26468 26475Yang, X.; Hu, X. T.; Wang, Q. X.; Xiong, J.; Yang, H. J.; Meng, X. C.; Tan, L. C.; Chen, L.; Chen, Y. W. Large-scale stretchable semiembedded copper nanowire transparent conductive films by an electrospinning template. ACS Appl. Mater. Interfaces 2017, 9, 26468−26475.
Transparent, flexible and low-resistive precision fabric electrode for organic solar cells
Phys. Status Solidi-R 2009 3 278 280Castro, F. A.; Chabrecek, P.; Hany, R.; Nuesch, F. Transparent, flexible and low-resistive precision fabric electrode for organic solar cells. Phys. Status Solidi-R 2009, 3, 278−280.
Flexible molecular-scale electronic devices
Nat. Nanotechnol. 2012 7 438 442Park, S.; Wang, G.; Cho, B.; Kim, Y.; Song, S.; Ji, Y.; Yoon, M. H.; Lee, T. Flexible molecular-scale electronic devices. Nat. Nanotechnol. 2012, 7, 438−442.
Mechanically robust all-polymer solar cells from narrow band gap acceptors with hetero-bridging atoms
Joule 2020 4 658 672Fan, Q.; Su, W.; Chen, S.; Kim, W.; Chen, X.; Lee, B.; Liu, T.; Méndez-Romero, U. A.; Ma, R.; Yang, T.; Zhuang, W.; Li, Y.; Li, Y.; Kim, T. S.; Hou, L.; Yang, C.; Yan, H.; Yu, D.; Wang, E. Mechanically robust all-polymer solar cells from narrow band gap acceptors with hetero-bridging atoms. Joule 2020, 4, 658−672.
Highly efficient flexible polymer solar cells with robust mechanical stability
Adv. Sci. 2019 6 1801180Tan, L.; Wang, Y.; Zhang, J.; Xiao, S.; Zhou, H.; Li, Y.; Chen, Y.; Li, Y. Highly efficient flexible polymer solar cells with robust mechanical stability. Adv. Sci. 2019, 6, 1801180.
Stretchable organic solar cells
Adv. Mater. 2011 23 1771 1775Lipomi, D. J.; Tee, B. C.; Vosgueritchian, M.; Bao, Z. Stretchable organic solar cells. Adv. Mater. 2011, 23, 1771−1775.
Toward mechanically robust and intrinsically stretchable organic solar cells: Evolution of photovoltaic properties with tensile strain
Solar Energy Mater. Solar Cells 2012 107 355 365Lipomi, D. J.; Chong, H.; Vosgueritchian, M.; Mei, J.; Bao, Z. Toward mechanically robust and intrinsically stretchable organic solar cells: Evolution of photovoltaic properties with tensile strain. Solar Energy Mater. Solar Cells 2012, 107, 355−365.
Novel cross-linked films from epoxy-functionalized conjugated polymer and amine based small molecule for the interface engineering of high-efficiency inverted polymer solar cells
Solar Ener. Mater. Solar Cells 2017 168 22 29Lin, K.; Wang, J.; Hu, Z.; Xu, R.; Liu, J.; Liu, X.; Xu, B.; Huang, F.; Cao, Y. Novel cross-linked films from epoxy-functionalized conjugated polymer and amine based small molecule for the interface engineering of high-efficiency inverted polymer solar cells. Solar Ener. Mater. Solar Cells 2017, 168, 22−29.
Water/alcohol soluble conjugated polymers as highly efficient electron transporting/injection layer in optoelectronic devices
Chem. Soc. Rev. 2010 39 2500 2521Huang, F.; Wu, H.; Cao, Y. Water/alcohol soluble conjugated polymers as highly efficient electron transporting/injection layer in optoelectronic devices. Chem. Soc. Rev. 2010, 39, 2500−2521.
Highly efficient inverted polymer solar cell by low temperature annealing of Cs2CO3 interlayer
Appl. Phys. Lett. 2008 92 173303Liao, H. H.; Chen, L. M.; Xu, Z.; Li, G.; Yang, Y. Highly efficient inverted polymer solar cell by low temperature annealing of Cs2CO3 interlayer. Appl. Phys. Lett. 2008, 92, 173303.
Synergistic optimization enables large-area flexible organic solar cells to maintain over 98% PCE of the small-area rigid devices
Adv. Mater. 2017 32 2005153Wang, G.; Zhang, J.; Yang, C.; Wang, Y.; Xing, Y.; Adil, M. A.; Yang, Y.; Tian, L.; Su, M.; Shang, W.; Lu, K.; Shuai, Z.; Wei, Z. Synergistic optimization enables large-area flexible organic solar cells to maintain over 98% PCE of the small-area rigid devices. Adv. Mater. 2017, 32, 2005153.
An inverted organic solar cell with an ultrathin Ca electron-transporting layer and MoO3 hole-transporting layer
Appl. Phys. Lett. 2009 95 153304Zhao, D. W.; Liu, P.; Sun, X. W.; Tan, S. T.; Ke, L.; Kyaw, A. K. K. An inverted organic solar cell with an ultrathin Ca electron-transporting layer and MoO3 hole-transporting layer. Appl. Phys. Lett. 2009, 95, 153304.
Toward an ideal polymer binder design for high-capacity battery anodes
J. Am. Chem. Soc. 2013 135 12048 12056Wu, M.; Xiao, X.; Vukmirovic, N.; Xun, S.; Das, P. K.; Song, X.; Olalde-Velasco, P.; Wang, D.; Weber, A. Z.; Wang, L. W.; Battaglia, V. S.; Yang, W.; Liu, G. Toward an ideal polymer binder design for high-capacity battery anodes. J. Am. Chem. Soc. 2013, 135, 12048−12056.
Origin of work function modification by ionic and amine-based interface layers
Adv. Mater. Interfaces 2014 1 1400189van Reenen, S.; Kouijzer, S.; Janssen, R. A. J.; Wienk, M. M.; Kemerink, M. Origin of work function modification by ionic and amine-based interface layers. Adv. Mater. Interfaces 2014, 1, 1400189.
Regular energetics at conjugated electrolyte/electrode modifier for organic electronics and their implications on design rules
Adv. Mater. Interfaces 2015 2 1400189Bao, Q.; Liu, X.; Wang, E.; Fang, J.; Gao, F.; Braun, S.; Fahlman, M. Regular energetics at conjugated electrolyte/electrode modifier for organic electronics and their implications on design rules. Adv. Mater. Interfaces 2015, 2, 1400189.
Triple dipole effect from self-assembled small-molecules for high performance organic photovoltaics
Adv. Mater. 2016 28 4852 4860Huang, L.; Chen, L.; Huang, P.; Wu, F.; Tan, L.; Xiao, S.; Zhong, W.; Sun, L.; Chen, Y. Triple dipole effect from self-assembled small-molecules for high performance organic photovoltaics. Adv. Mater. 2016, 28, 4852−4860.
n-Type conjugated electrolytes cathode interlayer with thickness-insensitivity for highly efficient organic solar cells
J. Mater. Chem. A 2017 5 13807 13816Xu, G.; Gao, L.; Xu, H.; Huang, L.; Xie, Y.; Cheng, X.; Li, Y.; Chen, L.; Chen, Y. n-Type conjugated electrolytes cathode interlayer with thickness-insensitivity for highly efficient organic solar cells. J. Mater. Chem. A 2017, 5, 13807−13816.
Low work-function poly(3,4-ethylenedioxylenethiophene): poly(styrene sulfonate) as electron-transport layer for high-efficient and stable polymer solar cells
Sci. Rep. 2015 5 12839Zhang, Y.; Chen, L.; Hu, X.; Zhang, L.; Chen, Y. Low work-function poly(3,4-ethylenedioxylenethiophene): poly(styrene sulfonate) as electron-transport layer for high-efficient and stable polymer solar cells. Sci. Rep. 2015, 5, 12839.
A versatile buffer layer for polymer solar cells: rendering surface potential by regulating dipole
Adv. Funct. Mater. 2015 25 3164 3171Zhong, W.; Chen, L.; Xiao, S.; Huang, L.; Chen, Y. A versatile buffer layer for polymer solar cells: rendering surface potential by regulating dipole. Adv. Funct. Mater. 2015, 25, 3164−3171.
Efficient polymer solar cells employing a non-conjugated small-molecule electrolyte, Nat
Photon. 2015 9 520 524Ouyang, X.; Peng, R.; Ai, L.; Zhang, X.; Ge, Z. Efficient polymer solar cells employing a non-conjugated small-molecule electrolyte, Nat. Photon. 2015, 9, 520−524.
Recent progress of organic photovoltaics for indoor energy harvesting
Nano Energy 2021 82 105770Xie, L.; Song, W.; Ge, J.; Tang, B.; Zhang, X.; Wu, T.; Ge, Z. Recent progress of organic photovoltaics for indoor energy harvesting. Nano Energy 2021, 82, 105770.
High-efficiency thermal-annealing-free organic solar cells based on an asymmetric acceptor with improved thermal and air stability
ACS Appl. Mater. Interfaces 2020 12 57271 57280Zhang, J.; Han, Y.; Zhang, W.; Ge, J.; Xie, L.; Xia, Z.; Song, W.; Yang, D.; Zhang, X.; Ge, Z. High-efficiency thermal-annealing-free organic solar cells based on an asymmetric acceptor with improved thermal and air stability. ACS Appl. Mater. Interfaces 2020, 12, 57271−57280.
Vertical stratification engineering for organic bulk-heterojunction devices
ACS Nano 2018 12 4440 4452Huang, L.; Wang, G.; Zhou, W.; Fu, B.; Cheng, X.; Zhang, L.; Yuan, Z.; Xiong, S.; Zhang, L.; Xie, Y.; Zhang, A.; Zhang, Y.; Ma, W.; Li, W.; Zhou, Y.; Reichmanis, E.; Chen, Y. Vertical stratification engineering for organic bulk-heterojunction devices. ACS Nano 2018, 12, 4440−4452.
Interface design for high-efficiency non-fullerene polymer solar cells
Energy Environ. Sci. 2017 10 1784 1791Sun, C.; Wu, Z.; Hu, Z.; Xiao, J.; Zhao, W.; Li, H. W.; Li, Q. Y.; Tsang, S. W.; Xu, Y. X.; Zhang, K.; Yip, H. L.; Hou, J.; Huang, F.; Cao, Y. Interface design for high-efficiency non-fullerene polymer solar cells. Energy Environ. Sci. 2017, 10, 1784−1791.
n-Type water/alcohol-soluble naphthalene diimide-based conjugated polymers for high-performance polymer solar cells
J. Am. Chem. Soc. 2016 138 2004 2013Wu, Z.; Sun, C.; Dong, S.; Jiang, X. F.; Wu, S.; Wu, H.; Yip, H. L.; Huang, F.; Cao, Y. n-Type water/alcohol-soluble naphthalene diimide-based conjugated polymers for high-performance polymer solar cells. J. Am. Chem. Soc. 2016, 138, 2004−2013.
Counterion-tunable n-type conjugated polyelectrolytes for the interface engineering of efficient polymer solar cells
J. Mater. Chem. A 2017 5 19447 19455Chen, Z.; Hu, Z.; Wu, Z.; Liu, X.; Jin, Y.; Xiao, M.; Huang, F.; Cao, Y. Counterion-tunable n-type conjugated polyelectrolytes for the interface engineering of efficient polymer solar cells. J. Mater. Chem. A 2017, 5, 19447−19455.
A self-organized poly(vinylpyrrolidone)-based cathode interlayer in inverted fullerene-free organic solar cells
Adv. Mater. 2019 31 1804657Yang, B.; Zhang, S.; Li, S.; Yao, H.; Li, W.; Hou, J. A self-organized poly(vinylpyrrolidone)-based cathode interlayer in inverted fullerene-free organic solar cells. Adv. Mater. 2019, 31, 1804657.